Femoral Neck System
Karl Stoffel, Christoph Sommer
The Femoral Neck System (FNS) was designed to address clinical challenges associated with the fixation of femoral neck fractures. The FNS implants form a fixed-angle gliding fixation device that allows for controlled collapse of the femoral neck, similar to existing dynamic hip screw systems. The lateral element is comprised of a small base plate with one or two locking hole options. Due to the small size of the base plate, a single plate barrel angle can cover the clear majority of caputcollumdiaphyseal (CCD) angles without major angulation and offset of the base plate on the lateral aspect of the femur. The barrel allows for gliding of the head elements, in this case the locked combination of bolt and antirotation screw, while simultaneously restricting rotation around the head-neck axis.
Clinical problem
Femoral neck fractures represent 50% of all reported hip fracture cases. Although most common among patients aged 65 and older and often with comorbidities such as osteoporosis, such fractures also affect the young patient population as a result of high energy trauma.
While total hip arthroplasty (THA) and hemiarthroplasty (HA) remain the preferred treatment options for displaced femoral neck fractures in the older population, internal fixation with anatomical reduction (either closed or open) is considered state of the art for younger patients. Sliding hip screws offer a higher mechanical strength over cannulated screws for the fixation of such fractures but are usually accompanied by higher blood loss due to the invasive approach that is required for insertion. Both implants have also been reported to result in a likelihood of lateral thigh pain due to implant protrusion. The Lower Extremity Expert Group has been working on a solution to combat such problems - the Femoral Neck System (FNS).
Clinical solution
FNS is designed for the minimal-invasive fixation of femoral neck fractures of all types. As a result of rotational and angular stable design features within the system, FNS aims to demonstrate a high resistance against varus collapse and femoral head rotation.
Bolt and antirotation screw
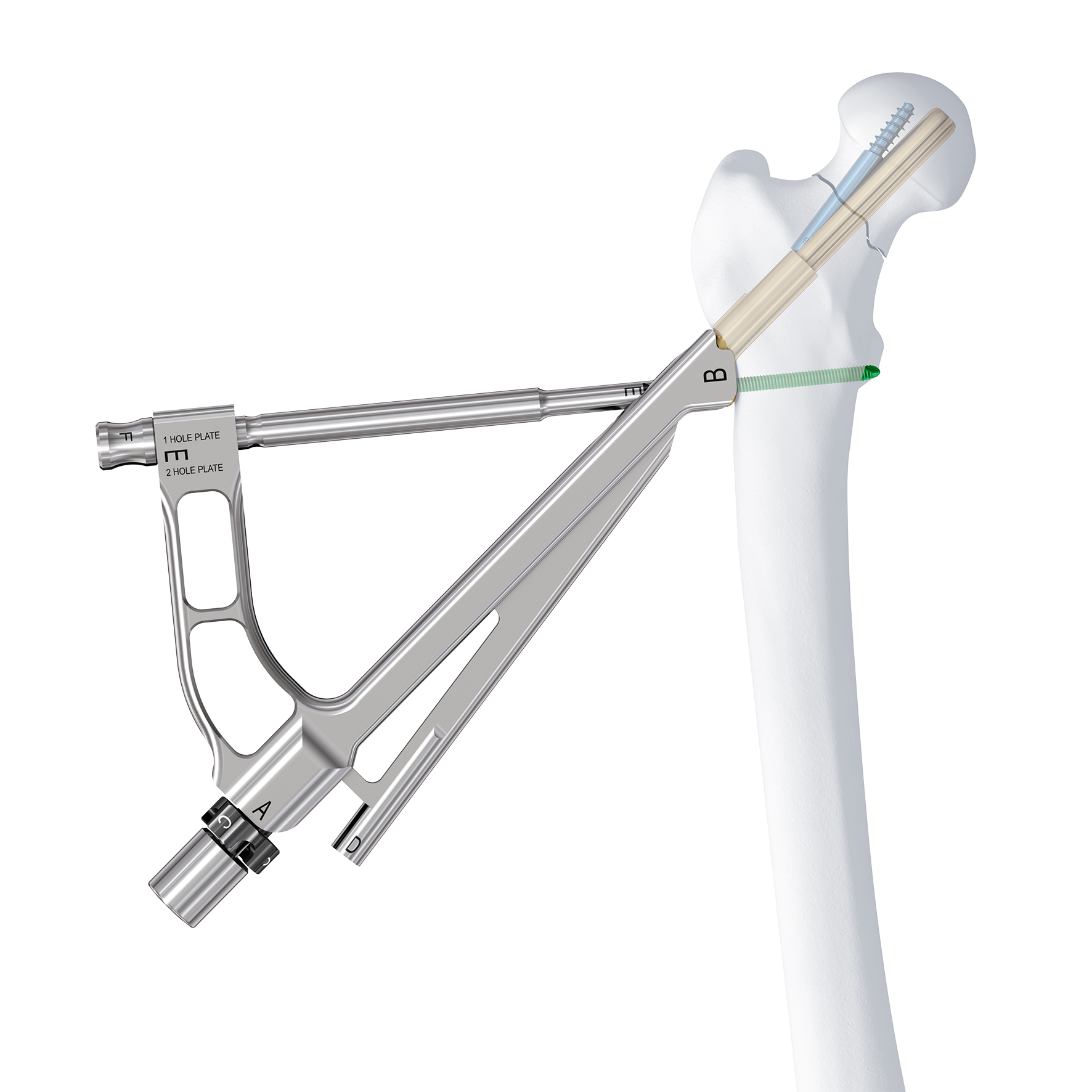
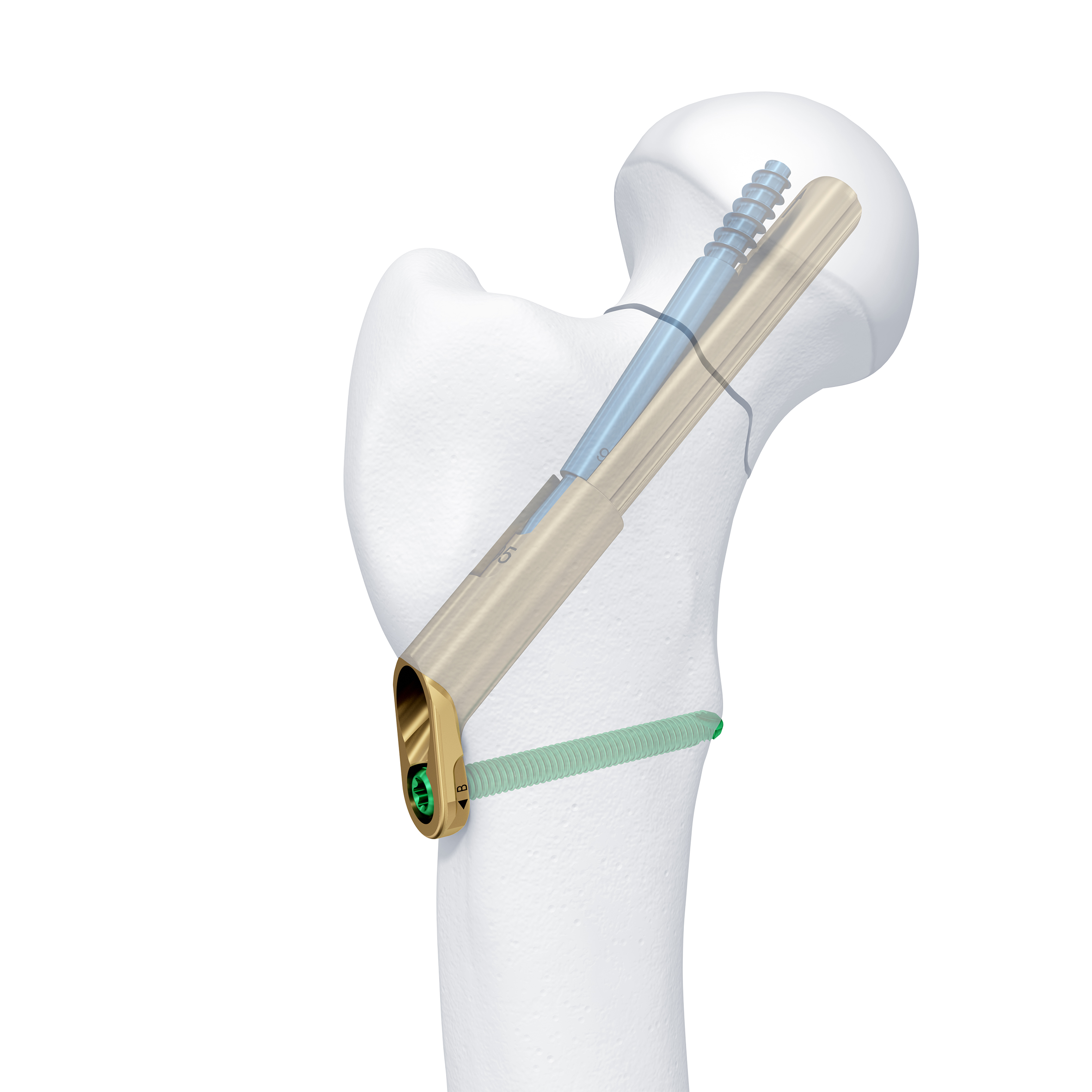
The unique rotational and angular stable design is achieved by two key elements of the system: the femoral neck bolt and the antirotation screw (ARScrew) (Fig 1).
The bolt forms the central load carrier for the femoral head fragment. Its blunt tip reduces the risk of cut-out in the articular space. The bolt glides freely within the barrel of the base plate while rotational motion is restricted. This allows controlled collapse of the head fragment of up to 20 mm. Most of this collapse occurs within the barrel of the base plate reducing lateral protrusion to a minimum. The ARScrew enhances the rotational stability of the head-neck fragment. It is locked with the bolt at a fixed angle and therefore glides assembled to the bolt in the barrel of the base plate. The ARScrew also has a blunt tip that reduces the risk of cut-out in the articular space. Unlike other systems that often demand the insertion of an antirotation screw separately, both implants in the FNS interconnect and work together to create a fixed angle construct with the benefits of a dynamic design. The bolt and ARScrew slide over a distance of 20 mm thereby enabling the controlled dynamic compression required for the fixation of femoral neck fractures.
Base plate design
The small base plate design of the FNS (Fig 2) results in an optimal implant footprint when compared with other sliding hip screw constructs. The ARScrew is inserted through the base plate and therefore avoids the need for additional soft-tissue dissection proximal to the plate. This facilitates a more minimally invasive surgical approach, which is an additional benefit of the FNS along with a reduced risk of lateral implant protrusion, lateral hip pain, and subsequent soft-tissue irritation.
Instrumentation
Procedural efficiency for femoral neck fractures has been shown to differ significantly based on the method of treatment selected. The surgical technique required for sliding hip screws has been described as technically difficult and is often associated with increased intraoperative blood loss when compared with fixation by mulitple screws. Subsequently, the intrumentation necessary for such demanding procedures should be intuitive with a minimization of surgical steps and maximization of procedural efficiency. The instruments for FNS have been designed for simplicity and ease of use. Implant insertion is achieved using one central guide wire and one primary instrument assembly (Fig 1)
Femoral Neck System A New Technique for Minimal Invasive Fixation of Femoral Neck Fractures
Biomechanical performance
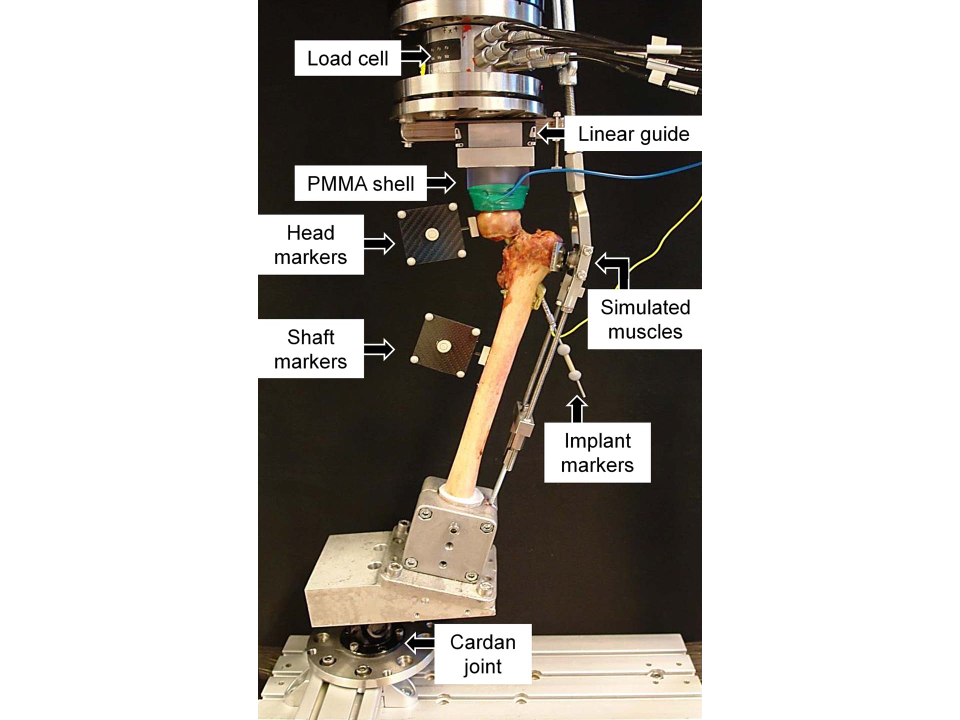
The AO Research Institute Davos investigated the biomechanical performance of the Femoral Neck System in comparison to the Dynamic Hip Screw (DHS) with an antirotation screw, DHS with blade, and three cannulated screws in the treatment of an unstable femoral neck fracture. The biomechanical performance of FNS was equal to both DHS implants and significantly better than three cannulated screws in terms of resistance to leg and neck shortening under cyclic loading. FNS was proven to be twice as strong as multiple screws against femoral neck and leg shortening. Please click here to read the full manuscript.
Hazards and labeling
Due to varying countries’ legal and regulatory approval requirements, consult the appropriate local product labeling for approved intended use of the products described on this website. All devices on this website are approved by the AO Technical Commission. For logistical reasons, these devices may not be available in all countries worldwide at the date of publication.
Legal restrictions
This work was produced by AO Foundation, Switzerland. All rights reserved by AO Foundation. This publication, including all parts thereof, is legally protected by copyright.
Any use, exploitation or commercialization outside the narrow limits set forth by copyright legislation and the restrictions on use laid out below, without the publisher‘s consent, is illegal and liable to prosecution. This applies in particular to photostat reproduction, copying, scanning or duplication of any kind, translation, preparation of microfilms, electronic data processing, and storage such as making this publication available on Intranet or Internet.
Some of the products, names, instruments, treatments, logos, designs, etc referred to in this publication are also protected by patents, trademarks or by other intellectual property protection laws (eg, “AO” and the AO logo are subject to trademark applications/registrations) even though specific reference to this fact is not always made in the text. Therefore, the appearance of a name, instrument, etc without designation as proprietary is not to be construed as a representation by the publisher that it is in the public domain.
Restrictions on use: The rightful owner of an authorized copy of this work may use it for educational and research purposes only. Single images or illustrations may be copied for research or educational purposes only. The images or illustrations may not be altered in any way and need to carry the following statement of origin “Copyright by AO Foundation, Switzerland”.
Check www.aofoundation.org/disclaimer for more information.
If you have any comments or questions on the articles or the new devices, please do not hesitate to contact us.
“approved by AO Technical Commission” and “approved by AO”
The brands and labels “approved by AO Technical Commission” and “approved by AO”, particularly "AO" and the AO logo, are AO Foundation's intellectual property and subject to trademark applications and registrations, respectively. The use of these brands and labels is regulated by licensing agreements between AO Foundation and the producers of innovation products obliged to use such labels to declare the products as AO Technical Commission or AO Foundation approved solutions. Any unauthorized or inadequate use of these trademarks may be subject to legal action.
AO ITC Innovations Magazine
Find all issues of the AO ITC Innovations Magazine for download here.
Innovation Awards
Recognizing outstanding achievements in development and fostering excellence in surgical innovation.