Expert Nailing System (EX Nail) Instrumentation
The Expert nailing system range of implants is state-of-the-art, with several built-in features that optimize anatomical design and improve locking options, both equally important for successful treatment with intramedullary nailing. However, a thorough review of existing nailing instrumentation has revealed several clinical challenges: difficulty in determining the correct entry point, complexity in obtaining adequate fracture reduction, limited bone visibility due to metallic instruments, limited readability of measuring devices, and loss of screws in soft tissues during insertion. The need for a large number of instruments, which are not compatible with all nail types, makes inventory control a problem for hospitals. The new Expert nailing system (EX Nail) Instrumentation has been developed to address these issues and to optimize functionality, ergonomics, user-friendliness, durability, and ease of cleaning.
The new EX Nail Instrumentation is intended to be used with the Expert Lateral Femoral Nail (LFN-EX), Expert Adolescent Lateral Femoral Nail (ALFN-EX), Expert Retrograde/Antegrade Femoral Nail (R/AFN-EX) and Expert Tibial Nail (TN-EX) and is comprised of the following instruments:
- Radiolucent Insertion Handles and Aiming Arms
- Opening Instruments, Wire Guides, and Protection Sleeves with Handle
- Intramedullary Reduction Tool with T Handle
- Screwdrivers / Interlock Screwdrivers
- Measuring Devices
- Scalpel Handle
- Connecting Screw for Insertion Handle
- Cutter for the Expert Tibial Nail
- Handle (large) with Quick Coupling
- Combined Hammer 500g
- Driving Cap for Insertion Handle
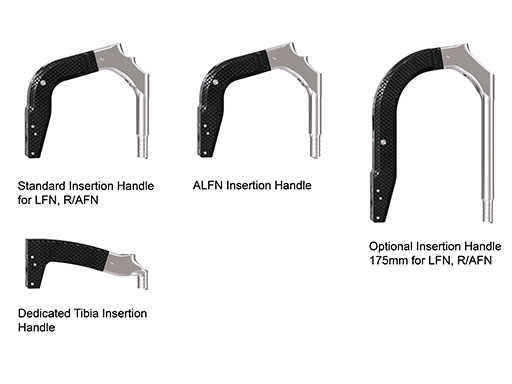
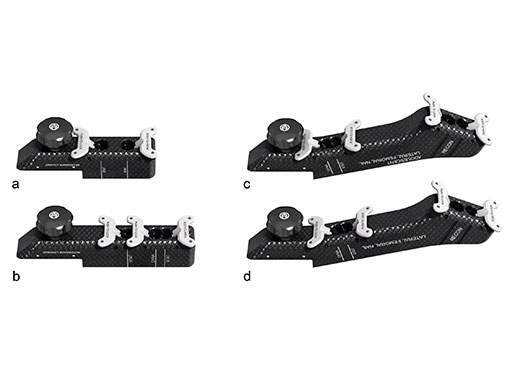
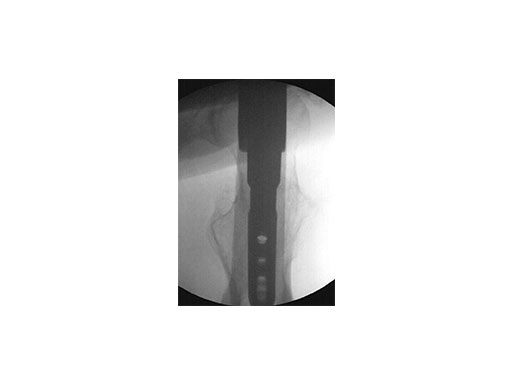
Use of metallic instruments limits the surgeon's view during fluoroscopy, resulting in difficulty visualizing the fracture and the proper positioning of the locking mechanisms in the bone. The new insertion handles have an increased radiolucent area (Fig 1). In the US, the Standard Insertion Handle is used for the LFN-EX, R/AFN-EX, and Expert Tibial Nail. The shorter Dedicated Tibia Insertion Handle is optional for tibia procedures. Together with the radiolucent aiming arms (Fig 2), the visualization of the relevant bony anatomy on intraoperative x-ray images is improved (Fig 3). The new aiming arms have built in retention mechanisms and cam locks, which allow true-locking of the protection sleeves to decrease the risk of accidental loosening of the parts during the procedure.
Wire guide
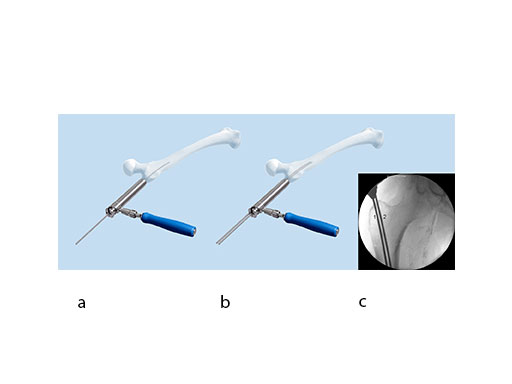
Finding the correct entry point and path of the K-wire by trial and error can be cumbersome and frustrating. The new wire guides have a center hole and one or two offset holes. If the initial K-wire has not been inserted through the proper entry point, a second K-wire can be correctly placed in reference to the first wire through one of the offset holes (4 mm and/ or 6 mm from the central hole) of the instrument (Fig 4). The wire guide can be rotated in the protection sleeve and locked into four set positions with 90 axial offsets. This guide aids the insertion of a K-wire at the correct entry point (Fig 4c) when the first wire has been placed in an offset position. Color-coding limits the possibility of mismatching the instruments.
Intramedullary Reduction Tool
Achievement of proper reduction, especially in femoral fractures, can be very challenging. A long reduction tool (Fig 5) has been developed, which has an anatomical curvature and a pointed fingertip to aid in fracture reduction.
The EX Nail Instrumentation has been developed based on surgeons needs in order to make the nailing procedure easier and more straight forward, to reduce OR time, and to improve results. The compatibility of the EX Nail Instruments also helps to reduce inventory and facilitates the OR set-up for a variety of procedures.
Hazards and labeling
Due to varying countries’ legal and regulatory approval requirements, consult the appropriate local product labeling for approved intended use of the products described on this website. All devices on this website are approved by the AO Technical Commission. For logistical reasons, these devices may not be available in all countries worldwide at the date of publication.
Legal restrictions
This work was produced by AO Foundation, Switzerland. All rights reserved by AO Foundation. This publication, including all parts thereof, is legally protected by copyright.
Any use, exploitation or commercialization outside the narrow limits set forth by copyright legislation and the restrictions on use laid out below, without the publisher‘s consent, is illegal and liable to prosecution. This applies in particular to photostat reproduction, copying, scanning or duplication of any kind, translation, preparation of microfilms, electronic data processing, and storage such as making this publication available on Intranet or Internet.
Some of the products, names, instruments, treatments, logos, designs, etc referred to in this publication are also protected by patents, trademarks or by other intellectual property protection laws (eg, “AO” and the AO logo are subject to trademark applications/registrations) even though specific reference to this fact is not always made in the text. Therefore, the appearance of a name, instrument, etc without designation as proprietary is not to be construed as a representation by the publisher that it is in the public domain.
Restrictions on use: The rightful owner of an authorized copy of this work may use it for educational and research purposes only. Single images or illustrations may be copied for research or educational purposes only. The images or illustrations may not be altered in any way and need to carry the following statement of origin “Copyright by AO Foundation, Switzerland”.
Check www.aofoundation.org/disclaimer for more information.
If you have any comments or questions on the articles or the new devices, please do not hesitate to contact us.
“approved by AO Technical Commission” and “approved by AO”
The brands and labels “approved by AO Technical Commission” and “approved by AO”, particularly "AO" and the AO logo, are AO Foundation's intellectual property and subject to trademark applications and registrations, respectively. The use of these brands and labels is regulated by licensing agreements between AO Foundation and the producers of innovation products obliged to use such labels to declare the products as AO Technical Commission or AO Foundation approved solutions. Any unauthorized or inadequate use of these trademarks may be subject to legal action.
AO ITC Innovations Magazine
Find all issues of the AO ITC Innovations Magazine for download here.
Innovation Awards
Recognizing outstanding achievements in development and fostering excellence in surgical innovation.