Vertebral Body Stent (VBS or Stentoplasty) and Vertecem V+
Roger Härtl, Jean Ouellet, Andreas Korge, Robert McGuire, Paul Heini
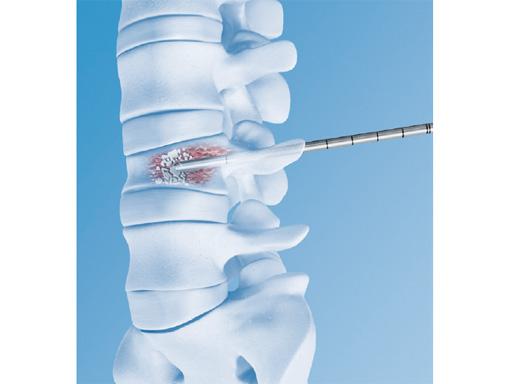
The vertebral body stent (VBS) system is a minimally invasive, percutaneous, reconstructive treatment for vertebral body fractures. The VBS system is intended for the reduction of painful vertebral compression fractures and/or creation of a void in cancellous bone in the spine for the treatment of levels ranging from T5-L5. It is intended to be used in combination with a legally marketed polymethylmethacrylate (PMMA)-based bone cement adequately indicated for use in vertebroplasty or kyphoplasty procedures.
Vertecem V+
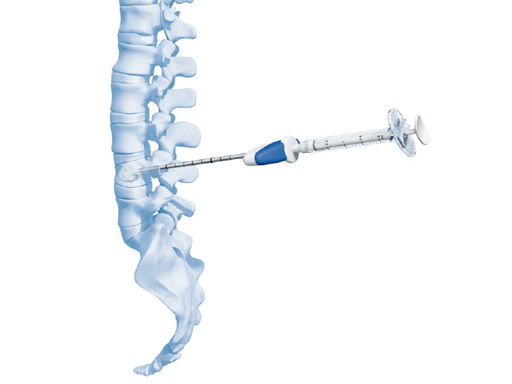
Vertecem V+ is for percutaneous injection of PMMA as filler material within (eg, stentoplasty) or without (vertebroplasty) an intervertebral framework. Many different products are available on the market to cover both solutions.
Vertecem V+ is unique because it:
- Is ready to use directly upon mixing (no need to wait for the cement to reach the right viscosity)
- Offers prolonged working time (less stress for the surgeon through reduced time constraints/the opportunity to perform multilevel interventions)
- Offers better visibility under x-ray through the incorporation of more radiopacifiers, which are also distributed more evenly inside the PMMA cement
The vertecem V+ syringe kit offers syringes in 5 x 2 mL and 8 x 2 mL sizes. The design features strong syringes, color coding for different volumes, and ergonomic handling.
The vertebroplasty needle kit features 8 gauge (blue), 10 gauge (yellow), and 12 gauge (green) diamond- and beveled-tip needles. It also offers an optional biopsy solution for 8 gauge and 10 gauge needles.
The design of this product achieves three goals:
- Long working time: (up to 27 minutes at room temperature) therefore less stress for the surgeon during the intervention and enough time for multilevel procedures.
- Increased initial viscosity: Ready to use directly upon mixing. Potentially lower risk for leakage and shorter operative time.
- Better x-ray visibility: Better control of cement flow and thus less risk of leakage.
Vertebral Body Stenting (VBS) System w/Balloon (VBB)
Hazards and labeling
Due to varying countries’ legal and regulatory approval requirements, consult the appropriate local product labeling for approved intended use of the products described on this website. All devices on this website are approved by the AO Technical Commission. For logistical reasons, these devices may not be available in all countries worldwide at the date of publication.
Legal restrictions
This work was produced by AO Foundation, Switzerland. All rights reserved by AO Foundation. This publication, including all parts thereof, is legally protected by copyright.
Any use, exploitation or commercialization outside the narrow limits set forth by copyright legislation and the restrictions on use laid out below, without the publisher‘s consent, is illegal and liable to prosecution. This applies in particular to photostat reproduction, copying, scanning or duplication of any kind, translation, preparation of microfilms, electronic data processing, and storage such as making this publication available on Intranet or Internet.
Some of the products, names, instruments, treatments, logos, designs, etc referred to in this publication are also protected by patents, trademarks or by other intellectual property protection laws (eg, “AO” and the AO logo are subject to trademark applications/registrations) even though specific reference to this fact is not always made in the text. Therefore, the appearance of a name, instrument, etc without designation as proprietary is not to be construed as a representation by the publisher that it is in the public domain.
Restrictions on use: The rightful owner of an authorized copy of this work may use it for educational and research purposes only. Single images or illustrations may be copied for research or educational purposes only. The images or illustrations may not be altered in any way and need to carry the following statement of origin “Copyright by AO Foundation, Switzerland”.
Check www.aofoundation.org/disclaimer for more information.
If you have any comments or questions on the articles or the new devices, please do not hesitate to contact us.
“approved by AO Technical Commission” and “approved by AO”
The brands and labels “approved by AO Technical Commission” and “approved by AO”, particularly "AO" and the AO logo, are AO Foundation's intellectual property and subject to trademark applications and registrations, respectively. The use of these brands and labels is regulated by licensing agreements between AO Foundation and the producers of innovation products obliged to use such labels to declare the products as AO Technical Commission or AO Foundation approved solutions. Any unauthorized or inadequate use of these trademarks may be subject to legal action.
AO ITC Innovations Magazine
Find all issues of the AO ITC Innovations Magazine for download here.
Innovation Awards
Recognizing outstanding achievements in development and fostering excellence in surgical innovation.