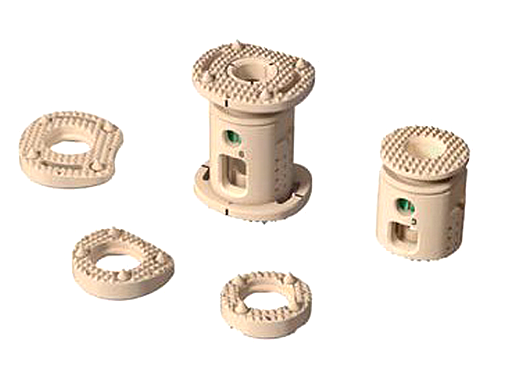
XRL Vertebral Body Replacement
The XRL Vertebral Body Replacement (VBR) implant is intended for use in the thoracolumbar spine (T1-L5) to replace a collapsed, damaged, or unstable vertebral body due to tumor or trauma (ie, fracture).
The XRL VBR has been designed to provide anterior spinal column support, even in the absence of fusion for a prolonged period. Due to the fact that its biomechanical characteristics (eg, modulus of elasticity) sit between the cancellous and cortical bone, the XRL VBR aids in stress distribution and load sharing. The implant is intended to be used with Synthes supplemental internal fixation systems (eg, USS, including MATRIX, Pangea, and TSLP).
The interior of the XRL VBR can be packed with bone (ie, autograft or allograft), and the PEEK implant's radiolucency gives surgeons the ability to assess tumor recurrence and fusion progress.
The implant encompasses modular endplates to accommodate multiple approach options. There are six endplate footprints that range from -10 to 20 degrees and construct heights that range from 22 to 145 mm, allowing the surgeon to customize the implant to better match patient anatomy.
An all-in-one spreader gives the surgeon the ability to insert, expand and lock implants all with one instrument.
Additionally, three solution options currently exist:
- Allograft
- Static VBRs in titanium and PEEK
- Expandable VBRs in titanium and PEEK.
However, use of the XRL VBR implant is contraindicated when there is active systemic infection, infection localized to the site of the proposed implantation, or when the patient has demonstrated allergy or foreign body sensitivity to any of the implant materials. Severe osteoporosis may also prevent adequate fixation, and therefore preclude the use of this or any other orthopaedic implant.
Conditions that place excessive stresses on bones and implants, such as severe obesity or degenerative diseases, are relative contraindications. The decision whether to use this device with patients with such conditions must be made by the physician, taking into account the risks versus the benefits to the patient.
Use of the implants is also relatively contraindicated in patients whose activity, mental capacity, mental illness, alcoholism, drug abuse, occupation, or lifestyle interferes with their ability to follow postoperative restrictions, potentially placing undue stress on the implant during bone healing, resulting in a higher risk of implant failure.
Case: Posterior-anterior en bloc spondylectomy
Case provided by Ziya L Gokaslan, Baltimore, USA
A 46-year-old man presented to an emergency department complaining of an acutely worsening pain that radiated from his lower back and buttock region down his left leg.
The patient had a two-year history of similar pain, unresponsive to physical therapy. The patient also complained of constipation and walking difficulty. a b He denied smoking or drug/alcohol use. His past medical and surgical history were unremarkable. Family history was noncontributory.
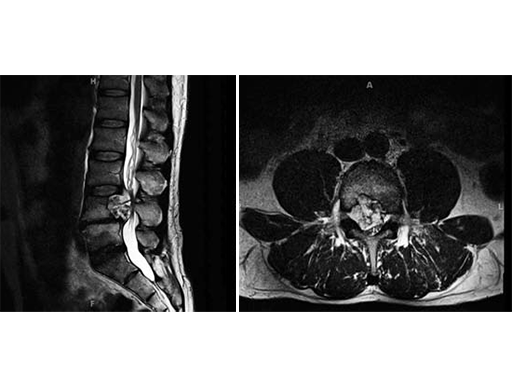
The patient was admitted for evaluation, and on MRI, was found to have a lesion at the L4 vertebral body, extending into the epidural space and compressing the cauda equine.
The patient was admitted for evaluation, and on MRI, was found to have a lesion at the L4 vertebral body, extending into the epidural space and compressing the cauda equine. The lesion was biopsied and found to be a chordoma, so he was transferred to the Neurosurgery department at Johns Hopkins Hospital for further management. His initial examination there was normal; motor strength 5/5 in all extremities, intact sensation, and reflexes present at 2+ throughout.
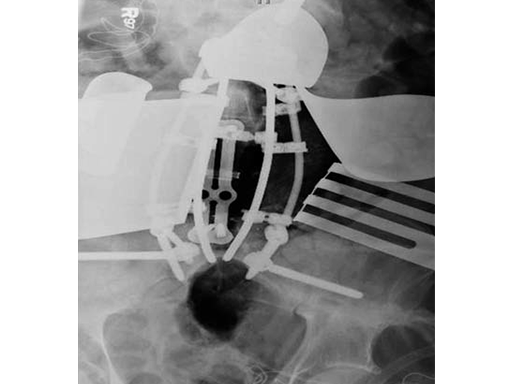
After discussing the risks of surgery, the patient consented to a staged posterior-anterior en bloc spondylectomy. The first stage involved a posterior lumbar pelvic exposure with dissection of the iliopsoas and lumbar plexus. Laminectomy of L3-L5 was performed, with instrumentation from L1 to the ilium bilaterally. Tomita saws were placed at the L3 and L5 disk space.
Stage two was a retroperitoneal exposure of L3-L5 with mobilation of the IVS, aorta, internal common illiac artery and veins, with en bloc resection of the tumor and partial L3 and L5 vertebrectomies.
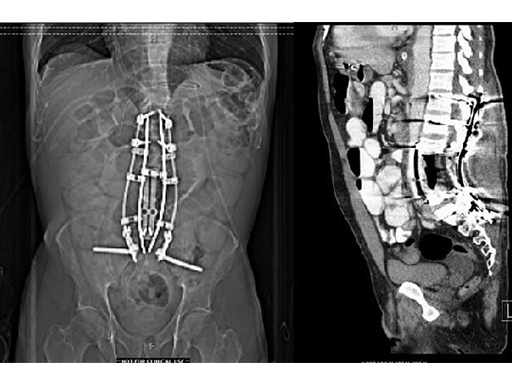
The vertebral body defect was reconstructed with the PEEK distractible cage, anterior plate, screws, and demineralized bone matrix. Final pathology revealed a true en bloc resection with negative margins.
The patient recovered well from surgery, but developed postoperative pneumonia and transient postoperative ileus. But at the time of discharge, the patient was ambulating well and was able to participate in active, inpatient rehabilitation.
Hazards and labeling
Due to varying countries’ legal and regulatory approval requirements, consult the appropriate local product labeling for approved intended use of the products described on this website. All devices on this website are approved by the AO Technical Commission. For logistical reasons, these devices may not be available in all countries worldwide at the date of publication.
Legal restrictions
This work was produced by AO Foundation, Switzerland. All rights reserved by AO Foundation. This publication, including all parts thereof, is legally protected by copyright.
Any use, exploitation or commercialization outside the narrow limits set forth by copyright legislation and the restrictions on use laid out below, without the publisher‘s consent, is illegal and liable to prosecution. This applies in particular to photostat reproduction, copying, scanning or duplication of any kind, translation, preparation of microfilms, electronic data processing, and storage such as making this publication available on Intranet or Internet.
Some of the products, names, instruments, treatments, logos, designs, etc referred to in this publication are also protected by patents, trademarks or by other intellectual property protection laws (eg, “AO” and the AO logo are subject to trademark applications/registrations) even though specific reference to this fact is not always made in the text. Therefore, the appearance of a name, instrument, etc without designation as proprietary is not to be construed as a representation by the publisher that it is in the public domain.
Restrictions on use: The rightful owner of an authorized copy of this work may use it for educational and research purposes only. Single images or illustrations may be copied for research or educational purposes only. The images or illustrations may not be altered in any way and need to carry the following statement of origin “Copyright by AO Foundation, Switzerland”.
Check www.aofoundation.org/disclaimer for more information.
If you have any comments or questions on the articles or the new devices, please do not hesitate to contact us.
“approved by AO Technical Commission” and “approved by AO”
The brands and labels “approved by AO Technical Commission” and “approved by AO”, particularly "AO" and the AO logo, are AO Foundation's intellectual property and subject to trademark applications and registrations, respectively. The use of these brands and labels is regulated by licensing agreements between AO Foundation and the producers of innovation products obliged to use such labels to declare the products as AO Technical Commission or AO Foundation approved solutions. Any unauthorized or inadequate use of these trademarks may be subject to legal action.
AO ITC Innovations Magazine
Find all issues of the AO ITC Innovations Magazine for download here.
Innovation Awards
Recognizing outstanding achievements in development and fostering excellence in surgical innovation.