Transpalatal Distractor System
Over the past decades, distraction osteogenesis has been popular for lengthening procedures in long bones and bone-segment transfer. Today, it is of increasing importance in the CMF field. During distraction woven bone forms in the distraction gap. The distraction's progress must be fast enough to prevent bony bridging and slow enough to permit the bone's differentiation. Distraction of 1 mm per day, in one to four steps, has been found to be adequate. An advantage of distraction osteogenesis is that it allows for reconstruction of skeletal deformities in a controlled fashion with the native bone [1].
Transverse maxillary hypoplasia [2] is a clinical entity characterized by diminished growth of the maxilla in the transverse dimension, leading to anterior and posterior crowding of the maxillary dentition. This constriction of the maxillary arch occurs spontaneously or in association with congenital cleft lip and palate deformities. Clinical manifestations include unilateral or bilateral palatal crossbite, dental rotation or tilting, and malocclusion. An adequate transverse maxillary dimension [3] is an important factor of stable occlusion, and it positively affects facial aesthetics. A narrow and V-shaped dental arch, dental crowding, a posterior crossbite, unaesthetic black buccal corridors upon smiling and maxillary transverse deficiency are generally interrelated. The underlying structural abnormalities may secondarily lead to gingivaperiosteal and periodontal disease, speech pathology, nasal airway obstruction, andwhen a cleft palate is presentalveolar ridge collapse and oronasal fistula. The corrective goal of expanding the transverse dimensions of the palate allows for normal position and function of the dentition, improvement in occlusion, airway and speech, and optimization of aesthetic appearance. Maxillary transverse deficiency and malocclusions can be corrected with slow orthodontic expansion, rapid palatal expansion, surgically assisted rapid palatal expansion, or two-segmented Le Fort I type osteotomy with expansion at different growth status [4].
Surgically assisted rapid palatal expansion takes advantage of bone formation at the maxillary edges of the midline while they are separated by an external force.
The amount of desired expansion is an important factor for selecting maxillary expansion in adults. In general, an orthodontist can camouflage transverse maxillary deficiency of less than 5 mm with orthopaedic or orthodontic forces alone. When the maxillary transverse deficiency is greater than 5 mm, surgical assistance is required.
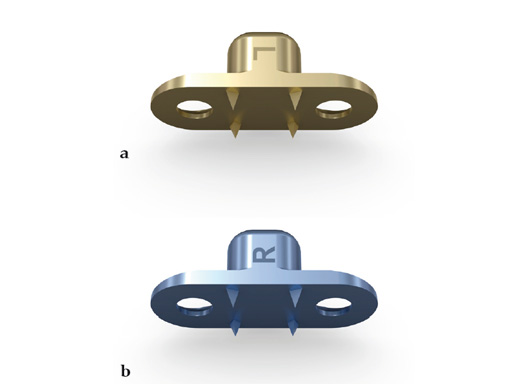
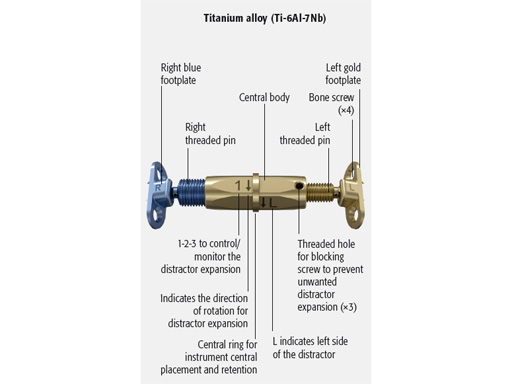
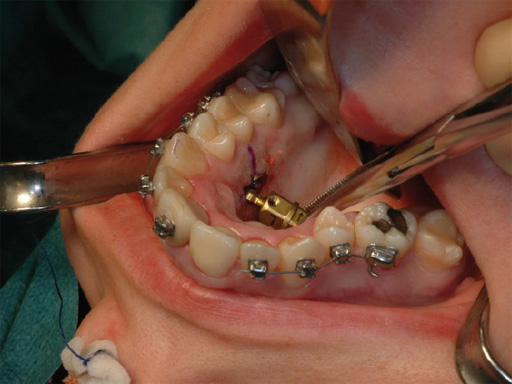
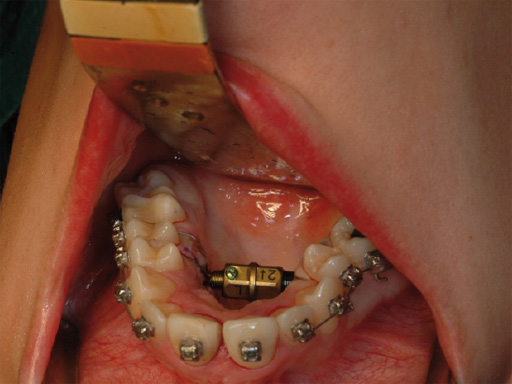
The transpalatal distractor system consists of a central body, plus left and right threaded pins that join with the respective footplate (Fig 3). They are fixed to the bone via four spikes on the bottom of the footplate (Fig 2).
Each rotation of the central body from one number to the next expands the transpalatal distractor by 0.33 mm. Therefore, a full rotation (360) would achieve an expansion of 1 mm.
Further rotation of the central body is prevented by inserting a blocking screw completely through one of the three threaded holes on the footplate's left side (Fig 3).
Recommendations
Ensure before the procedure that the safety wires are in the operating room. The preferred surgeon position relative to the patient's body while implanting the distractor is the top of the patient head position, since it facilitates facial symmetry observation. The bigger diameter screws are also preferred.
References
1 Prein J (1997) Manual of Internal Fixation in the Cranio-Facial Skeleton: Techniques Recommended by the AO/Asif Maxillofacial Group. Berlin Heidelberg New York: Springer-Verlag.
2 Vyas RM, Jarrahy R, Sisodia M, et al (2009) Bone-borne palatal distraction to correct the constricted cleft maxilla. J Craniofac Surg; 20(3):733736.
3 Gnbay T, Akay MC, Gnbay S, et al (2008) Transpalatal distraction using bone-borne distractor: clinical observations and dental and skeletal changes. J Oral Maxillofac Surg; 66(12):25032514.
4 Mommaerts MY (1999) Transpalatal distraction as a method of maxillary expansion. Br J Oral Maxillofac Surg; 37(4):268272.
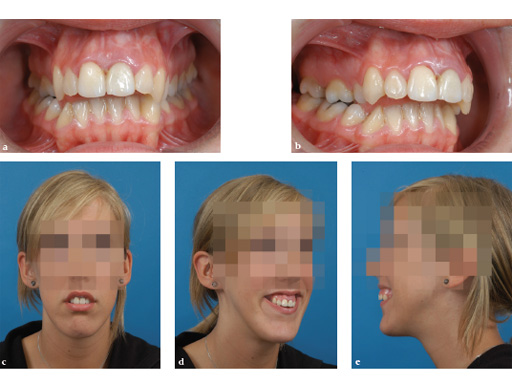
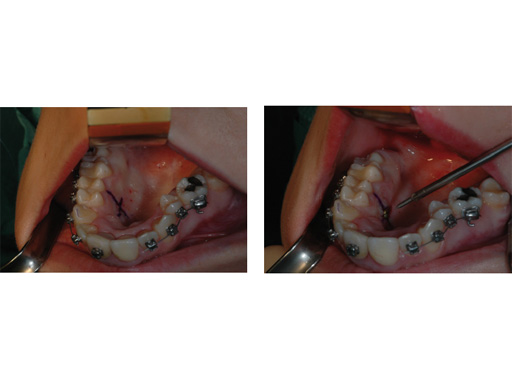
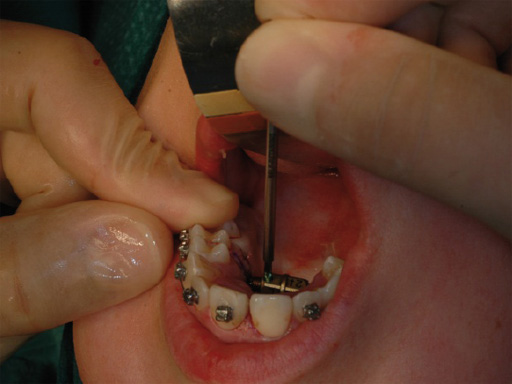
A 17-year-old girl with a long face and vertical maxillary excess. Class II malocclusion, narrow maxilla, hypoplastic cheekbones, and nasal deformity. The steps shown in Fig 1Fig 6 show a normal implantation procedure of the transpalatal distractor following surgical planning and standard osteotomy.
Case provided by Maurice Mommaerts, Bruges, Belgium
Postoperative distraction is achieved using an activation instrument that joins with the distractor central body. A rotation from one number to the next etched on the central body, expands the distractor 0.33 mm. Activation is recommended 0.33 mm once per day. After distraction is complete, the distractor is blocked again with the blocking screw during the consolidation period
Hazards and labeling
Due to varying countries’ legal and regulatory approval requirements, consult the appropriate local product labeling for approved intended use of the products described on this website. All devices on this website are approved by the AO Technical Commission. For logistical reasons, these devices may not be available in all countries worldwide at the date of publication.
Legal restrictions
This work was produced by AO Foundation, Switzerland. All rights reserved by AO Foundation. This publication, including all parts thereof, is legally protected by copyright.
Any use, exploitation or commercialization outside the narrow limits set forth by copyright legislation and the restrictions on use laid out below, without the publisher‘s consent, is illegal and liable to prosecution. This applies in particular to photostat reproduction, copying, scanning or duplication of any kind, translation, preparation of microfilms, electronic data processing, and storage such as making this publication available on Intranet or Internet.
Some of the products, names, instruments, treatments, logos, designs, etc referred to in this publication are also protected by patents, trademarks or by other intellectual property protection laws (eg, “AO” and the AO logo are subject to trademark applications/registrations) even though specific reference to this fact is not always made in the text. Therefore, the appearance of a name, instrument, etc without designation as proprietary is not to be construed as a representation by the publisher that it is in the public domain.
Restrictions on use: The rightful owner of an authorized copy of this work may use it for educational and research purposes only. Single images or illustrations may be copied for research or educational purposes only. The images or illustrations may not be altered in any way and need to carry the following statement of origin “Copyright by AO Foundation, Switzerland”.
Check www.aofoundation.org/disclaimer for more information.
If you have any comments or questions on the articles or the new devices, please do not hesitate to contact us.
“approved by AO Technical Commission” and “approved by AO”
The brands and labels “approved by AO Technical Commission” and “approved by AO”, particularly "AO" and the AO logo, are AO Foundation's intellectual property and subject to trademark applications and registrations, respectively. The use of these brands and labels is regulated by licensing agreements between AO Foundation and the producers of innovation products obliged to use such labels to declare the products as AO Technical Commission or AO Foundation approved solutions. Any unauthorized or inadequate use of these trademarks may be subject to legal action.
AO ITC Innovations Magazine
Find all issues of the AO ITC Innovations Magazine for download here.
Innovation Awards
Recognizing outstanding achievements in development and fostering excellence in surgical innovation.