Synapse 4.0
Posterior cervical fusion (PCF) surgery is often needed when patients require treatment of some typical spinal conditions, including degenerative disc disease, spondylosis, spinal stenosis, fracture, and tumor. Additionally, it can be an essential surgical option for revisions of seudarthrosis or failed previous surgery.
The goals of posterior cervical spine fusion surgery are to:
- Treat nerve and/or spinal cord impingement through decompression
- Achieve stabilization of spinal segments after fracture or trauma
- Correct deformity and maintain alignment
The implants and instruments available for surgery have continued to improve over time. Early techniques for posterior stabilization included use of wire or cable around affected bony structures, such as the lamina and/or spinous process. Posterior plate and screw constructs offered some additional stability and reduced potential complications, such as loosening of wiring, although screw placement and plate alignment remained difficult in many patients. The further development of multiangle screw and rod constructs offered surgeons the advantages of stable fixation and added flexibility for optimal placement.
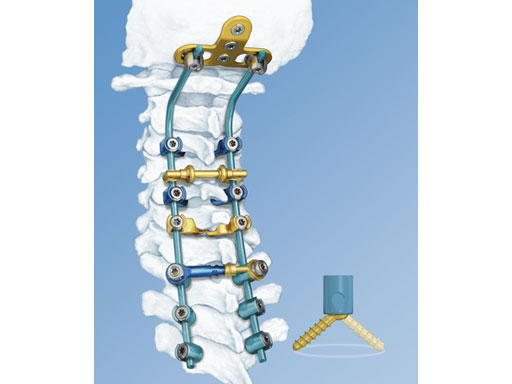
Comprehensive instrumentation for occipital cervical thoracic surgery
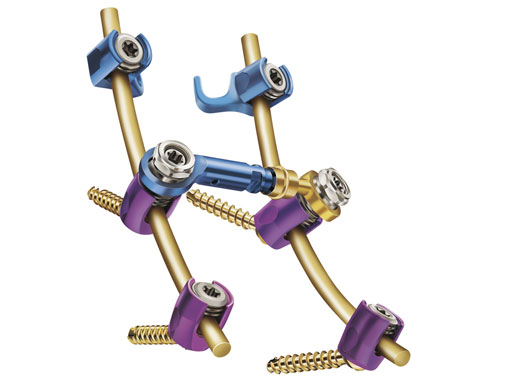
The synapse system is an enhanced set of instruments and implants, including clamps, variable-axis screws, hooks, transconnectors, transverse bars and rods, designed for posterior stabilization of the upper spine. The implants provide the flexibility required to accommodate variations in patient anatomy.
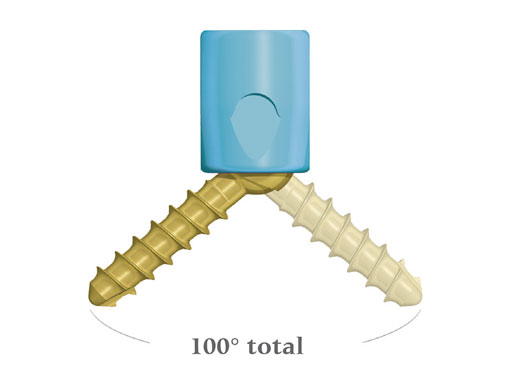
Key to achieving this flexibility is the polyaxial screw implant. With angulation in all directions, the implant accommodates the most demanding surgical constructs, without the need for favored-angled or biased screws. The locking cap utilizes a square-thread design to eliminate cross-threading during final tightening.
Synapse is available in two rod diameter sizes of 3.5 and 4.0 mm, and the new synapse 4.0 system allows for interchangeable use between both 3.5 and 4.0 mm diameter rods. This allows the surgeon to decide intraoperatively on the rod stiffness and strength, depending on the anatomy of the patient and the indication.
The 4.0 mm titanium alloy rod [Ti 6Al 7Nb] is 50% stronger and 70% stiffer than the existing 3.5 mm titanium alloy rods. It is ideal for surgical indications, such as:
- Crossing the occipitocervical [OC] and/or cervicothoracic [CT] junctions
- Severe trauma cases resulting in gross spinal instability
- Tumor situations where significant bone resection has destabilized the spine segments
The system includes a top-loading transconnector which attaches to the polyaxial screws to provide additional construct torsional rigidity when required.
One of the best instrumentation features of the system is the simple and efficient threaded screwdriver which eliminates screw toggle during insertion and mechanically disengages the screw to eliminate sticking of the driver in the screw recess.
The synapse system is fully compatible with the OC fusion systems to provide comprehensive instrumentation throughout the occipital cervical thoracic [OCT] region.
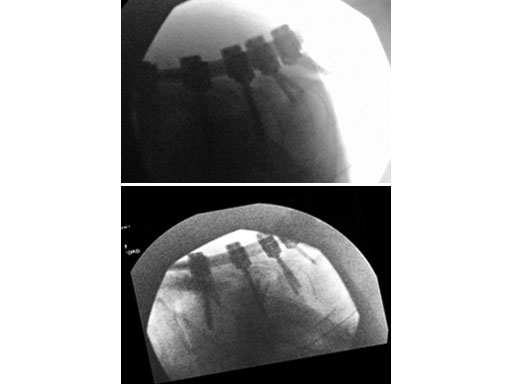
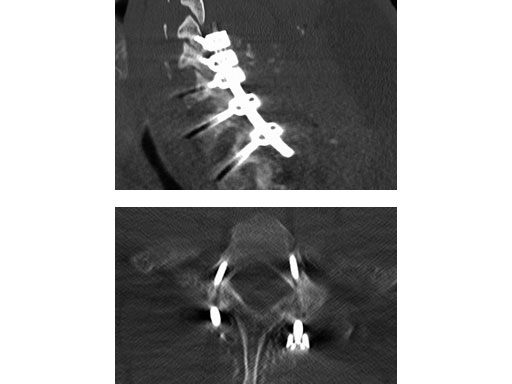
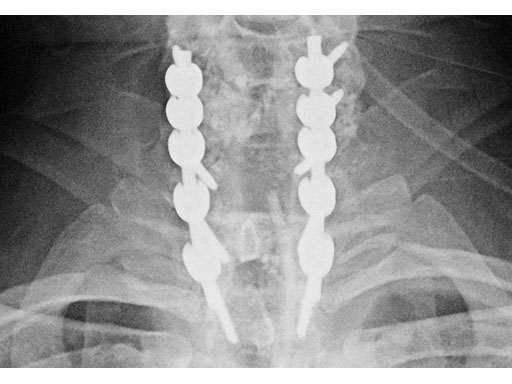
(Case provided by Rick Bransford, Seattle, USA)
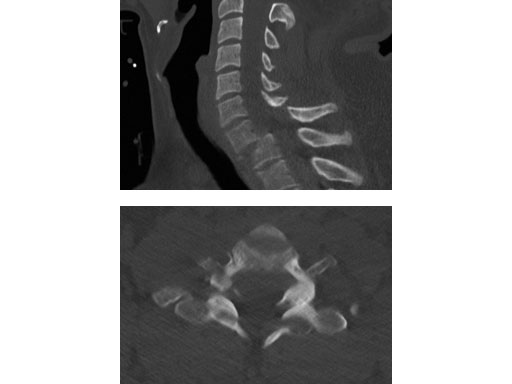
A 28-year-old, 109 kg man was involved in a high-speed motor vehicle collision with C7-T1 jumped facet and T1 teardrop fracture. The patient had ASIA E with motor score of 100. He had failed closed reduction in the emergency room then was taken to OR emergently for open reduction and C5-T2 posterior segmental instrumentation with synapse 4.0. The patient did well postoperatively with no evidence of collapse or failure.
Synapse 4.0 was the ideal solution to treat this injury. This was a heavy patient with a highly unstable injury at the cervicothoracic junction. Synapse 4.0 enabled stabilization of his injury with a rigid construct. It has all the benefits of the 3.5 system but is more rigid and allowed use of one system as opposed to using the 3.5 system and a 6.0 rod system with the nuances of a tapered rod. The author was able to place C5 and C6 lateral mass screws utilizing the Magerl technique and C7, T1, and T2 pedicle screws.
Hazards and labeling
Due to varying countries’ legal and regulatory approval requirements, consult the appropriate local product labeling for approved intended use of the products described on this website. All devices on this website are approved by the AO Technical Commission. For logistical reasons, these devices may not be available in all countries worldwide at the date of publication.
Legal restrictions
This work was produced by AO Foundation, Switzerland. All rights reserved by AO Foundation. This publication, including all parts thereof, is legally protected by copyright.
Any use, exploitation or commercialization outside the narrow limits set forth by copyright legislation and the restrictions on use laid out below, without the publisher‘s consent, is illegal and liable to prosecution. This applies in particular to photostat reproduction, copying, scanning or duplication of any kind, translation, preparation of microfilms, electronic data processing, and storage such as making this publication available on Intranet or Internet.
Some of the products, names, instruments, treatments, logos, designs, etc referred to in this publication are also protected by patents, trademarks or by other intellectual property protection laws (eg, “AO” and the AO logo are subject to trademark applications/registrations) even though specific reference to this fact is not always made in the text. Therefore, the appearance of a name, instrument, etc without designation as proprietary is not to be construed as a representation by the publisher that it is in the public domain.
Restrictions on use: The rightful owner of an authorized copy of this work may use it for educational and research purposes only. Single images or illustrations may be copied for research or educational purposes only. The images or illustrations may not be altered in any way and need to carry the following statement of origin “Copyright by AO Foundation, Switzerland”.
Check www.aofoundation.org/disclaimer for more information.
If you have any comments or questions on the articles or the new devices, please do not hesitate to contact us.
“approved by AO Technical Commission” and “approved by AO”
The brands and labels “approved by AO Technical Commission” and “approved by AO”, particularly "AO" and the AO logo, are AO Foundation's intellectual property and subject to trademark applications and registrations, respectively. The use of these brands and labels is regulated by licensing agreements between AO Foundation and the producers of innovation products obliged to use such labels to declare the products as AO Technical Commission or AO Foundation approved solutions. Any unauthorized or inadequate use of these trademarks may be subject to legal action.
AO ITC Innovations Magazine
Find all issues of the AO ITC Innovations Magazine for download here.
Innovation Awards
Recognizing outstanding achievements in development and fostering excellence in surgical innovation.