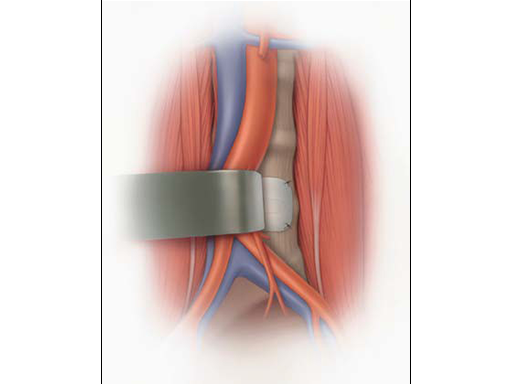
Scout Vessel Guard
Anterior spine surgery has been evolving since its inception in the early 1900s. Many technique improvements have helped address the challenge of mobilizing the anterior abdominal muscles, peritoneal sac, and great vessels, which is necessary to access the disc space. Protecting these sensitive vessels still remains a major challenge for this approach, especially during surgical revisions.
The ability to protect sensitive vessels during a primary anterior spinal surgery is of utmost importance. It is particularly desirable to limit contact between these vessels and any implanted device and/or inflammatory graft materials.
The Scout Vessel Guard has been designed with this very protection in mind. The guard is placed over the implanted materials, residing between the anterior spine and adjacent vessels, and provides improved protection to sensitive areas. The Scout Vessel Guard is a permanent implant. In the event that a revision surgery of the anterior spine is needed, the guard will be present and is able to be clearly visualized and identified. The presence of the Scout Vessel Guard then also helps the surgeon locate important surgical landmarks during challenging cases.
The Scout Vessel Guard is made of Hydrogel, a water-swollen, crosslinked polymer structure that can be produced to achieve various geometries and ranges of material properties. This product was developed to provide exceptional handling characteristics, minimal pore size, maximal surface smoothness, and easy size customization of the one and two level footprints offered. The cryogenic hydrogel formulation used in the Scout Vessel Guard is approximately 75% water, with the balance being a novel blend of mostly polyvinyl alcohol (PVA) and a small amount of polyvinyl pyrrolidone (PVP). This hydrophilic material is packaged and provided hydrated, requiring minimal prep time in the OR.
Hazards and labeling
Due to varying countries’ legal and regulatory approval requirements, consult the appropriate local product labeling for approved intended use of the products described on this website. All devices on this website are approved by the AO Technical Commission. For logistical reasons, these devices may not be available in all countries worldwide at the date of publication.
Legal restrictions
This work was produced by AO Foundation, Switzerland. All rights reserved by AO Foundation. This publication, including all parts thereof, is legally protected by copyright.
Any use, exploitation or commercialization outside the narrow limits set forth by copyright legislation and the restrictions on use laid out below, without the publisher‘s consent, is illegal and liable to prosecution. This applies in particular to photostat reproduction, copying, scanning or duplication of any kind, translation, preparation of microfilms, electronic data processing, and storage such as making this publication available on Intranet or Internet.
Some of the products, names, instruments, treatments, logos, designs, etc referred to in this publication are also protected by patents, trademarks or by other intellectual property protection laws (eg, “AO” and the AO logo are subject to trademark applications/registrations) even though specific reference to this fact is not always made in the text. Therefore, the appearance of a name, instrument, etc without designation as proprietary is not to be construed as a representation by the publisher that it is in the public domain.
Restrictions on use: The rightful owner of an authorized copy of this work may use it for educational and research purposes only. Single images or illustrations may be copied for research or educational purposes only. The images or illustrations may not be altered in any way and need to carry the following statement of origin “Copyright by AO Foundation, Switzerland”.
Check www.aofoundation.org/disclaimer for more information.
If you have any comments or questions on the articles or the new devices, please do not hesitate to contact us.
“approved by AO Technical Commission” and “approved by AO”
The brands and labels “approved by AO Technical Commission” and “approved by AO”, particularly "AO" and the AO logo, are AO Foundation's intellectual property and subject to trademark applications and registrations, respectively. The use of these brands and labels is regulated by licensing agreements between AO Foundation and the producers of innovation products obliged to use such labels to declare the products as AO Technical Commission or AO Foundation approved solutions. Any unauthorized or inadequate use of these trademarks may be subject to legal action.
AO ITC Innovations Magazine
Find all issues of the AO ITC Innovations Magazine for download here.
Innovation Awards
Recognizing outstanding achievements in development and fostering excellence in surgical innovation.