Norian Drillable
Michiel Verhofstad, Christian Matula
Norian drillable is a synthetic ceramic, injectable, moldable, and biocompatible bone-void filler indicated for tibial plateau, distal radius, calcaneal, and intertrochanteric fractures.
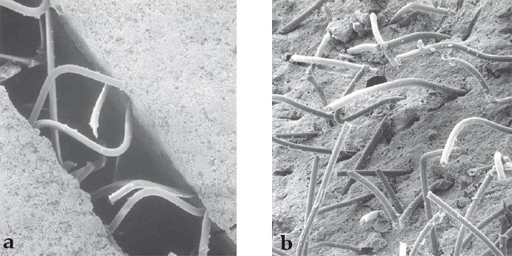
Norian drillable is a calcium-phosphate-based bone-void filler, based on the same ceramic phase as Norian SRS. It has been enhanced with the addition of chopped fibers as a reinforcing phase, and sodium hyaluronate as a flow promoter. The bioabsorbable polymer fibers, which comprise approximately 2% by weight of the cured material, are degraded in the body by hydrolysis, lose their strength in approximately 4 months, and are eliminated over time. The ceramic component is slowly remodeled over a period of years, at a rate depending on the metabolic activity of the host bone. When fully cured, the composition formed closely approximates the mineral phase of bone.
Norian drillable is intended to be placed into bony voids either before or after final fracture fixation. It allows drilling, tapping, and screw placement through the filler at any time even after the setting process is completed ("reduce → fill → fix"). Of course, Norian drillable can also be used after completion of the osteosynthesis in the same way as conventional Norian SRS ("reduce → fix → fill"). Once the material is set, it acts as a temporary medium that provides mechanical support during the healing process. This is of particular relevance in elderly patients with metaphyseal fractures with bone loss, eg, due to impaction. Please note that the screws should not be placed near the edge of the cement as this may cause a fracture of the bone-void filler.
Norian drillable can be delivered either as an injectable paste that is mixed with an automatic mixer or as a fast-set putty which is manually mixed with a cup and spatula. Both are available in amounts of 3.5 and 10 cc.
Norian drillable as injectable paste is prepared outside the sterile field with a mechanical rotary mixer and injected via a sterile syringe and needle. After mixing, the material forms a smooth, viscous paste that remains injectable for approximately 5 minutes at 18-23°C / 64-73°F. At body temperature (37°C / 98.6°F), Norian drillable begins to harden after 2 minutes and sets in approximately 10 minutes. If a tourniquet is used, the hardening is delayed, but can be enhanced using warm saline. Norian drillable reaches its ultimate compressive strength in 24 hours.
Norian drillable as fast-set putty is supplied in two containers: the mixing cup holds sterile powder and the solution syringe holds the liquid mixing solution. The moldable putty is mixed by hand with cup and spatula supplied in the kit within the sterile field and delivered by hand or another instrument. After mixing, the resultant putty material can be manipulated for 2 minutes at 18-23°C / 64-73°F. At body temperature (37°C / 98.6°F), Norian drillable fast-set putty begins to harden after 2 minutes of implantation and sets in approximately 36 minutes. Norian drillable fast-set putty reaches its ultimate compressive strength in 24 hours.
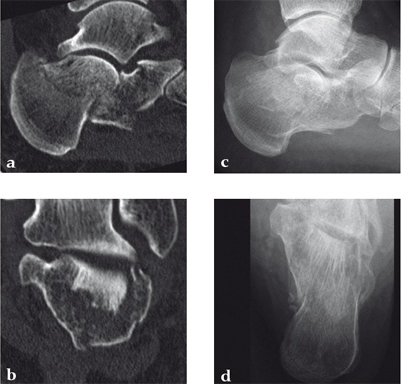
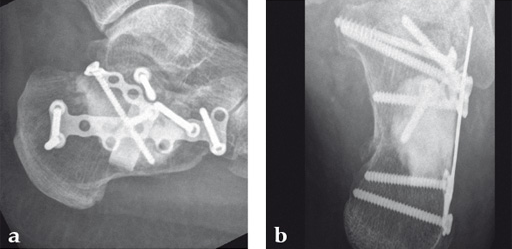
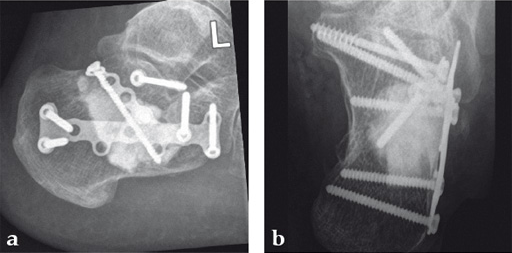
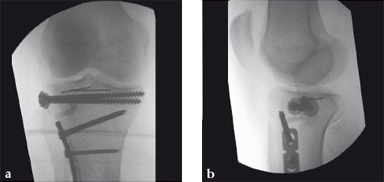
Case 1: A 70-year-old man sustained a Sanders IIA fracture of his left calcaneus.
(Case provided by Michiel Verhofstad, Tilburg, The Netherlands)
After open reduction a gap was left due to impaction of the osteoporotic metaphyseal bone. To support preliminary maintenance of the primary reduction a block of calcium phosphate was introduced beneath the posterior facet. The rest of the gap was filled with Norian drillable. After hardening plate osteosynthesis was performed. Two screws were placed through the fiber-enhanced calcium phosphate. Weight bearing was started 6 weeks later. After 6 months the fracture was healed without secondary loss of reduction. Note that at that time the degradation of the void filler is visible.
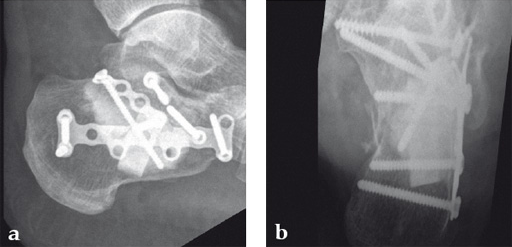
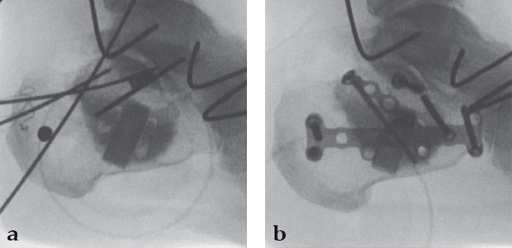
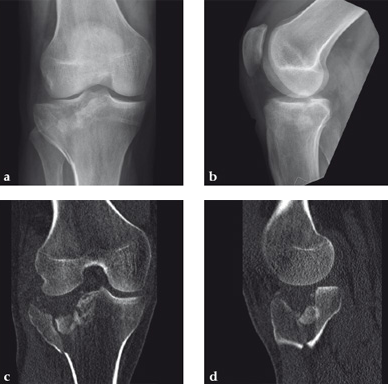
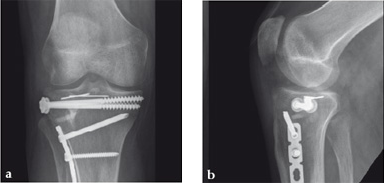
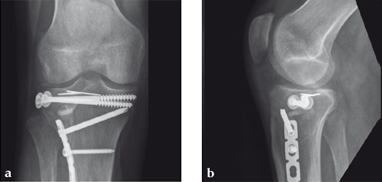
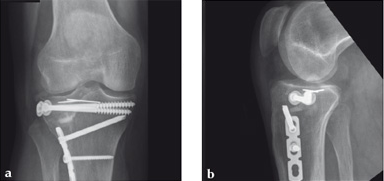
Case 2: A 38-year-old man sustained a lateral tibia plateau fracture (Müller AO Classification 41-B.3/Schatzker type II).
(Case provided by Michiel Verhofstad, Tilburg, The Netherlands)
After opening the lateral wedge, the osteochondral fragment was reduced and maintained with two K-wires. A gap beneath this fragment was left. Subsequently, a 3.2 mm hole was drilled in the lateral wedge using an inside-out technique. Then the lateral fragment was reduced. A 3-hole buttress plate, followed by two subchondral compression screws were used for final fracture fixation. Finally, Norian drillable was injected in the gap through the predrilled hole. Weight bearing was started after 6 weeks. At 6 months the fracture had healed anatomically and the patient was complaint-free.
Hazards and labeling
Due to varying countries’ legal and regulatory approval requirements, consult the appropriate local product labeling for approved intended use of the products described on this website. All devices on this website are approved by the AO Technical Commission. For logistical reasons, these devices may not be available in all countries worldwide at the date of publication.
Legal restrictions
This work was produced by AO Foundation, Switzerland. All rights reserved by AO Foundation. This publication, including all parts thereof, is legally protected by copyright.
Any use, exploitation or commercialization outside the narrow limits set forth by copyright legislation and the restrictions on use laid out below, without the publisher‘s consent, is illegal and liable to prosecution. This applies in particular to photostat reproduction, copying, scanning or duplication of any kind, translation, preparation of microfilms, electronic data processing, and storage such as making this publication available on Intranet or Internet.
Some of the products, names, instruments, treatments, logos, designs, etc referred to in this publication are also protected by patents, trademarks or by other intellectual property protection laws (eg, “AO” and the AO logo are subject to trademark applications/registrations) even though specific reference to this fact is not always made in the text. Therefore, the appearance of a name, instrument, etc without designation as proprietary is not to be construed as a representation by the publisher that it is in the public domain.
Restrictions on use: The rightful owner of an authorized copy of this work may use it for educational and research purposes only. Single images or illustrations may be copied for research or educational purposes only. The images or illustrations may not be altered in any way and need to carry the following statement of origin “Copyright by AO Foundation, Switzerland”.
Check www.aofoundation.org/disclaimer for more information.
If you have any comments or questions on the articles or the new devices, please do not hesitate to contact us.
“approved by AO Technical Commission” and “approved by AO”
The brands and labels “approved by AO Technical Commission” and “approved by AO”, particularly "AO" and the AO logo, are AO Foundation's intellectual property and subject to trademark applications and registrations, respectively. The use of these brands and labels is regulated by licensing agreements between AO Foundation and the producers of innovation products obliged to use such labels to declare the products as AO Technical Commission or AO Foundation approved solutions. Any unauthorized or inadequate use of these trademarks may be subject to legal action.
AO ITC Innovations Magazine
Find all issues of the AO ITC Innovations Magazine for download here.
Innovation Awards
Recognizing outstanding achievements in development and fostering excellence in surgical innovation.