Fracture Plates for Condylar Neck and Base
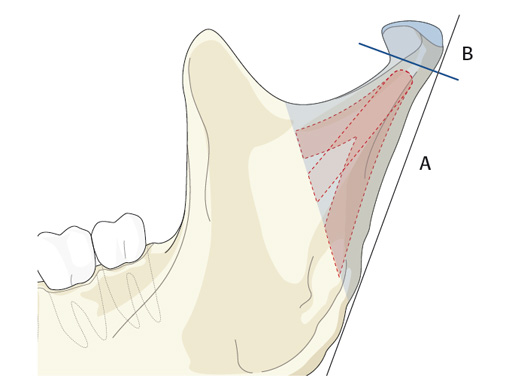
Fractures in the condylar region of the mandible present a specific challenge, ie, it can be difficult to access the region surgically due to the presence of the main stem of the facial nerve within the overlying soft tissue.
The most common solution for fractures in the neck and base region of the mandible is either closed treatment or various linear plating approaches, such as adaptation plates. When internal fixation is used a two-plate technique offers better stability than a one-plate technique. In response to a growing number of requests for improved treatment in this region, a series of plates have been developed with specific designs to treat such fractures and if possible to overcome such issues. The first two plates are now available.
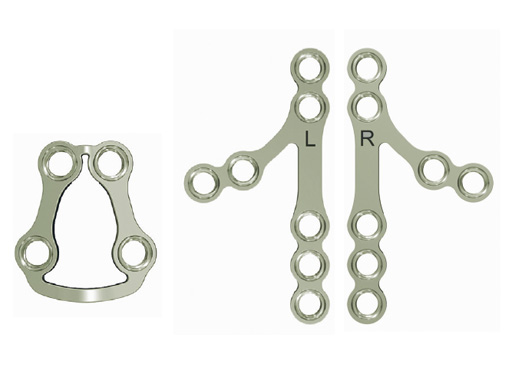
Trapezoidal Plate
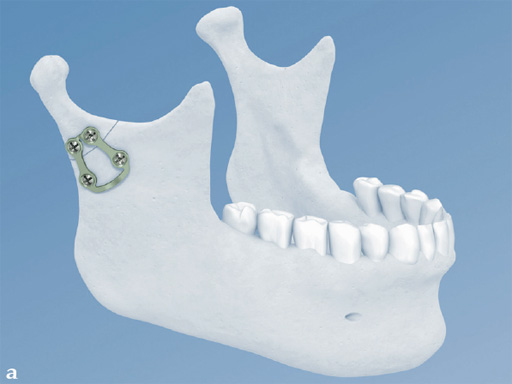
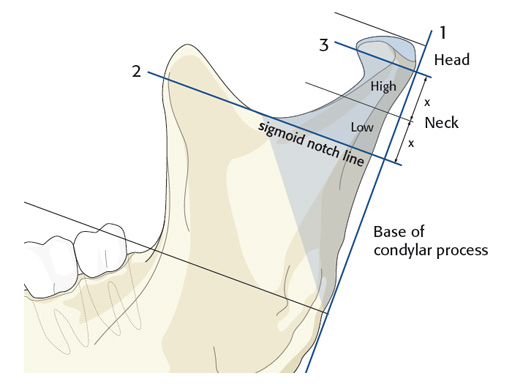
The trapezoidal plate is designed to fit the region of the condylar base and provides improved strength and stability over previous single-bar plate designs, thereby eliminating the need to place two plates. It is precontoured to account for the curvature in the transition zone between the base of the condylar process and the adjacent neck. The location of screw holes enables plates to straddle the mandibular foramen and the adjacent canal inlet, thereby avoiding accidental nerve injury. The trapezoidal plate can be applied using external and transoral surgical approaches. If required a transbuccal cannula can be centered in the countersunk screw holes for proper drilling alignment.
Lambda Plate
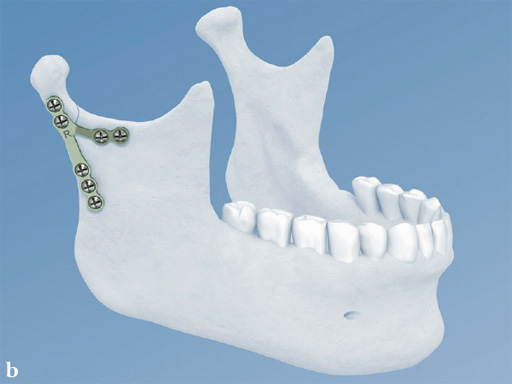
The lambda plate comes in a left and right version. It emulates a two-plate technique as its specific shape and 7-hole design with the width of a single plate at the top segment allows the surgeon to advance the lambda plate high up into the very narrow zone of the condylar neck just below the head. The fixation arms straddle the mandibular canal to avoid injury risk of the inferior alveolar nerve.
The lambda plate can be placed using retromandibular or submandibular surgical approaches. For positioning, the straight 5-hole segment is placed parallel to the posterior ramus border aligned with the condylar head. If required the anterior arm may be bent to fit the bony surface below the sigmoid notch.
Both plates are based on the matrix mandible system platform. Therefore, they are compatible with the existing matrix instrumentation and use the established color-coding for easy identification in the operating room. Both are 1.0 mm thick and malleable (green-grey color coding) and manufactured from commercially pure titanium.
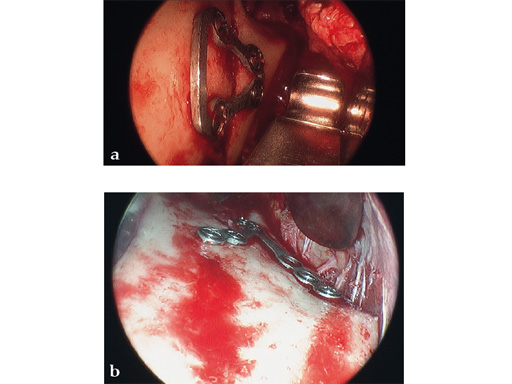
Case 1
Case provided by Celso Palmieri, Shreveport, LA, USA
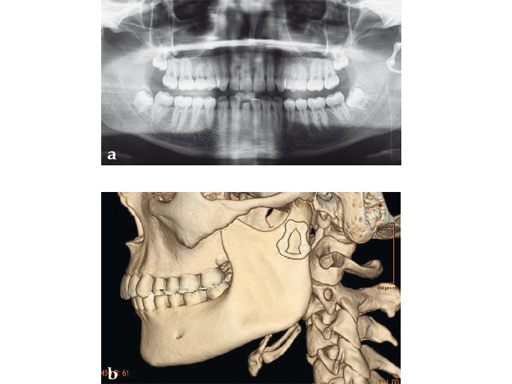
Case 2
Case provided by Michael Rasse, Innsbruck, Austria
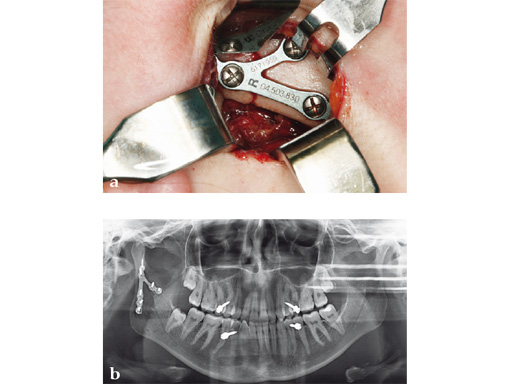
Case 3
Case provided by Carl-Peter Cornelius, Mnchen, Germany
Hazards and labeling
Due to varying countries’ legal and regulatory approval requirements, consult the appropriate local product labeling for approved intended use of the products described on this website. All devices on this website are approved by the AO Technical Commission. For logistical reasons, these devices may not be available in all countries worldwide at the date of publication.
Legal restrictions
This work was produced by AO Foundation, Switzerland. All rights reserved by AO Foundation. This publication, including all parts thereof, is legally protected by copyright.
Any use, exploitation or commercialization outside the narrow limits set forth by copyright legislation and the restrictions on use laid out below, without the publisher‘s consent, is illegal and liable to prosecution. This applies in particular to photostat reproduction, copying, scanning or duplication of any kind, translation, preparation of microfilms, electronic data processing, and storage such as making this publication available on Intranet or Internet.
Some of the products, names, instruments, treatments, logos, designs, etc referred to in this publication are also protected by patents, trademarks or by other intellectual property protection laws (eg, “AO” and the AO logo are subject to trademark applications/registrations) even though specific reference to this fact is not always made in the text. Therefore, the appearance of a name, instrument, etc without designation as proprietary is not to be construed as a representation by the publisher that it is in the public domain.
Restrictions on use: The rightful owner of an authorized copy of this work may use it for educational and research purposes only. Single images or illustrations may be copied for research or educational purposes only. The images or illustrations may not be altered in any way and need to carry the following statement of origin “Copyright by AO Foundation, Switzerland”.
Check www.aofoundation.org/disclaimer for more information.
If you have any comments or questions on the articles or the new devices, please do not hesitate to contact us.
“approved by AO Technical Commission” and “approved by AO”
The brands and labels “approved by AO Technical Commission” and “approved by AO”, particularly "AO" and the AO logo, are AO Foundation's intellectual property and subject to trademark applications and registrations, respectively. The use of these brands and labels is regulated by licensing agreements between AO Foundation and the producers of innovation products obliged to use such labels to declare the products as AO Technical Commission or AO Foundation approved solutions. Any unauthorized or inadequate use of these trademarks may be subject to legal action.
AO ITC Innovations Magazine
Find all issues of the AO ITC Innovations Magazine for download here.
Innovation Awards
Recognizing outstanding achievements in development and fostering excellence in surgical innovation.