ETN PROtect (Expert Tibia Nail, with Gentamicin Coating)
Michael Raschke, Gerhard Schmidmaier, Martin Lucke, Britt Wildemann, Thomas Fuchs
Bony infections of the tibia pose a serious threat to all patients treated for tibial injuries. These infections may lead to prolonged hospitalization, revision procedures, amputation or even death. In order to reduce the risk of deep surgical infection, the Expert Tibial Nail PROtect, including its cannulation, is coated with a thin layer of Gentamicin-loaded polymer. The coating is abrasion resistant and will withstand most of the forces occurring during nail insertion. The antibiotic coating of the nail releases antibiotic into the area surrounding the implant to reduce the infection risk.
Despite improvements in the prophylactic and the therapeutic measures, soft-tissue damage and consecutive infection remain the major hurdles in the healing process of long bone fractures. Osteomyelitis is a consequence of local contamination by germs combined with a local and/or systemic immunodeficiency. Additionally, blood supply in the traumatized bone is disturbed which means systemically applied antibiotics cannot reach the fracture site. Consequently, this may lead to chronic osteomyelitis. Once local osteomyelitis has become manifest, removal of the implant and aggressive debridement of the infected bone as well as the soft tissue in combination with heavy local and systemic antibiotic therapy often represent the only option for effective treatment.
Bacteria introduced during the insertion of surgical implants from the lower layers of the patient's own skin, which are not reached by skin disinfection, or at the time of injury in open fractures, are in competition with the patient's immune system. This is often described as "a race for the surface". Certain bacteria can colonize the surface of an implant and form a protective biofilm composed of proteins and polysaccharides, protecting the bacteria from the patient's immune system as well as from the effects of systemically applied antibiotics.
An antibiotic coating on an implant could prevent colonization of the implant's surface. In addition, a coating with high local antimicrobial concentrations could protect the site of injury and avoid side effects resulting from long-term high-dose systemic antibiotic therapy.
The Expert Tibial Nail (ETN) PROtect has a fully resorbable coating which consists of a polylactide (PDLLA) carrier containing gentamicin sulphate (Fig 1) to prevent implant colonization by bacteria. Gentamicin, an antibiotic from the aminoglycoside family, was chosen due to its broad antibacterial spectrum, the bactericidal effect (nonproliferating bacteria), its synergistic effect in combination with cephalosporins, and because it is well established for local application in orthopedics (bone cement, PMMA beads, collagen sponges). Gentamicin has a long and successful clinical track record in orthopedic and trauma surgery, and despite its widespread use, in particular in bone cements, the rates of resistance against gentamicin have remained stable or have been decreasing in recent years. The total amount of antibiotic contained on one implant ranges from around 15-60 mg, depending on the size of the implant. The gentamicin is released from the coating immediately after implantation with an initial burst that achieves a high peak concentration in the first hours and fades out over a period of a few weeks.
After coating, the implant is packaged and sterilized by gamma irradiation and delivered sterile to the clinics. The ETN PROtect can be used in extended indications, such as very proximal or very distal metaphyseal tibial fractures. In addition, the new implant may find its indication in revision surgeries or in patients where the immune system is compromised.
The mechanical properties of the nail are not affected by the coating. Tests on human specimens and plastic bones proved the coating to be resistant to the abrasive forces present during insertion into a narrow and moist bone canal. The surgical technique does not differ from the regular standard of care in ETN implantation.
Case provided by Gerhard Schmidmaier, Heidelberg, Germany
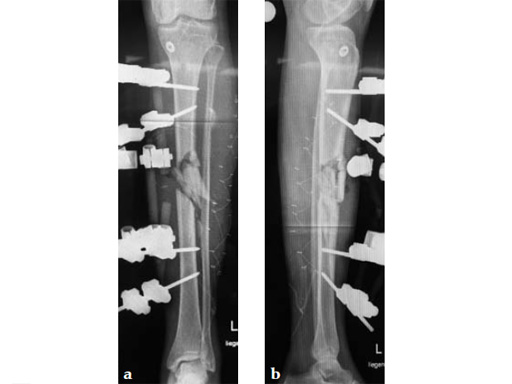
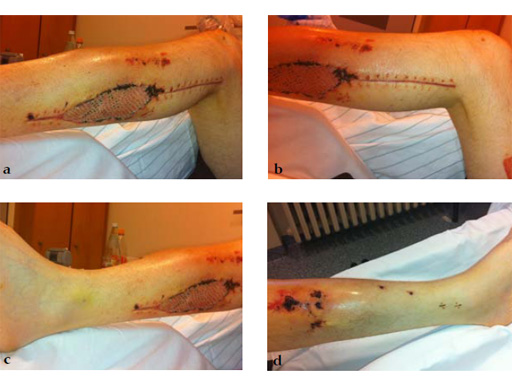
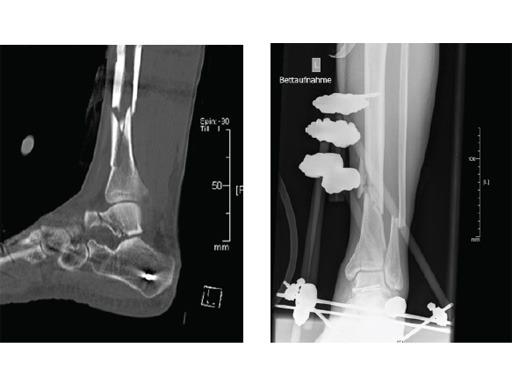
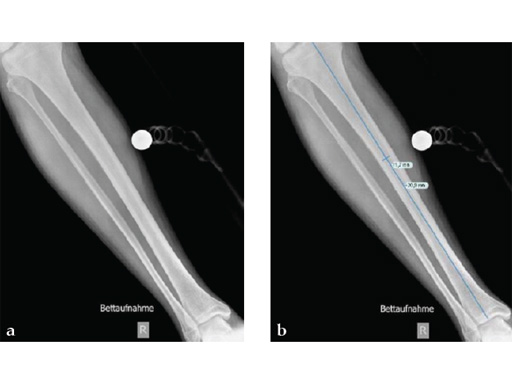
Clinical example of the first patient treated with the new ETN PROtect.
Case 1: A 33-year-old man with a third degree open fracture of his left tibia was initially treated with an external fixator and unilateral compartment release. He had undergone ACL reconstruction in the past.
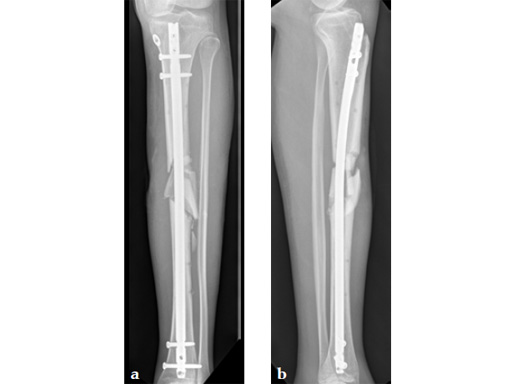
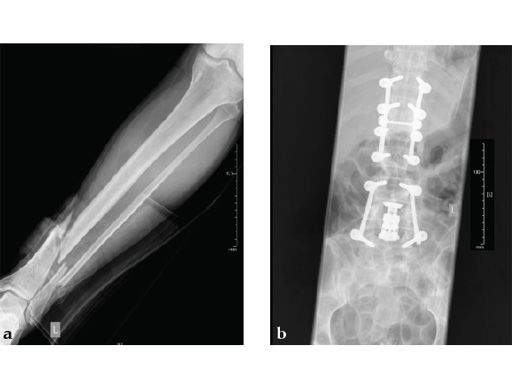
Case provided by Michael Raschke, Münster, Germany
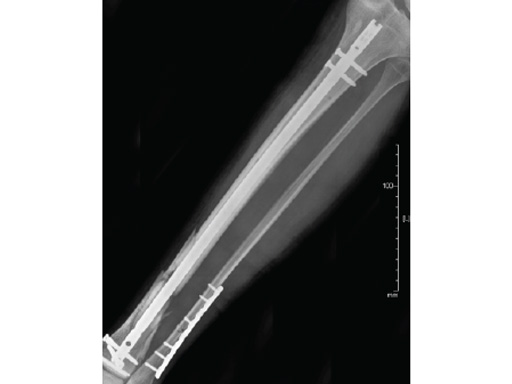
Case 2: Grade II open distal tibial fracture in a polytraumatized 52-year-old man.
Biomaterials in Infected Non-Unions
Hazards and labeling
Due to varying countries’ legal and regulatory approval requirements, consult the appropriate local product labeling for approved intended use of the products described on this website. All devices on this website are approved by the AO Technical Commission. For logistical reasons, these devices may not be available in all countries worldwide at the date of publication.
Legal restrictions
This work was produced by AO Foundation, Switzerland. All rights reserved by AO Foundation. This publication, including all parts thereof, is legally protected by copyright.
Any use, exploitation or commercialization outside the narrow limits set forth by copyright legislation and the restrictions on use laid out below, without the publisher‘s consent, is illegal and liable to prosecution. This applies in particular to photostat reproduction, copying, scanning or duplication of any kind, translation, preparation of microfilms, electronic data processing, and storage such as making this publication available on Intranet or Internet.
Some of the products, names, instruments, treatments, logos, designs, etc referred to in this publication are also protected by patents, trademarks or by other intellectual property protection laws (eg, “AO” and the AO logo are subject to trademark applications/registrations) even though specific reference to this fact is not always made in the text. Therefore, the appearance of a name, instrument, etc without designation as proprietary is not to be construed as a representation by the publisher that it is in the public domain.
Restrictions on use: The rightful owner of an authorized copy of this work may use it for educational and research purposes only. Single images or illustrations may be copied for research or educational purposes only. The images or illustrations may not be altered in any way and need to carry the following statement of origin “Copyright by AO Foundation, Switzerland”.
Check www.aofoundation.org/disclaimer for more information.
If you have any comments or questions on the articles or the new devices, please do not hesitate to contact us.
“approved by AO Technical Commission” and “approved by AO”
The brands and labels “approved by AO Technical Commission” and “approved by AO”, particularly "AO" and the AO logo, are AO Foundation's intellectual property and subject to trademark applications and registrations, respectively. The use of these brands and labels is regulated by licensing agreements between AO Foundation and the producers of innovation products obliged to use such labels to declare the products as AO Technical Commission or AO Foundation approved solutions. Any unauthorized or inadequate use of these trademarks may be subject to legal action.
AO ITC Innovations Magazine
Find all issues of the AO ITC Innovations Magazine for download here.
Innovation Awards
Recognizing outstanding achievements in development and fostering excellence in surgical innovation.