Computer assisted surgery for spinal instrumentation
Introduction
Spinal surgery inherently carries the potential of injury to spinal cord, nerves, and important vascular structures. There is general agreement among surgeons that imaging techniques are essential for safe and accurate placement of spinal instrumentation regardless of the complexity of spinal surgery, the anatomical region, and the level of training and comfort-level of the individual surgeon. Traditionally this has involved the use of x-ray or image intensification guidance either as a control at the end of a procedure or for active guidance throughout surgery.
Recently, stereotactic 2-D or 3-D imaging techniques have been developed and have gained acceptance in disciplines, such as cranial neurosurgery and certain orthopaedic procedures. Computer-assisted surgery (CAS) uses navigation systems to improve visibility to the surgical field and increase the accuracy of surgery and instrumentation placement by virtually linking the perated bony anatomy with pre- or intraoperative imaging studies, usually CT scans. The use of CAS has first been described for spinal instrumentation placement in the mid-1990s [14] and the different types of CAS have been reviewed in a recent AO publication [5]. However, CAS could potentially improve the way spinal surgery is performed. The purpose of this article is to review the basic concepts and current application of CAS within spinal surgery.
Types of computer-assisted surgery
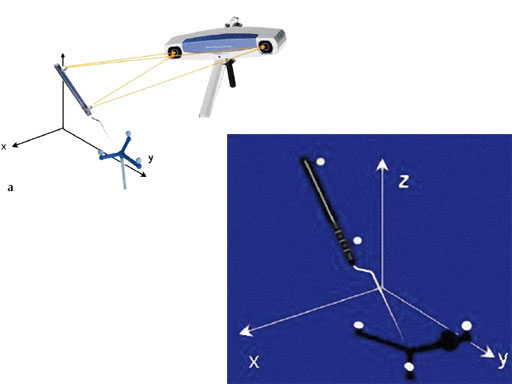
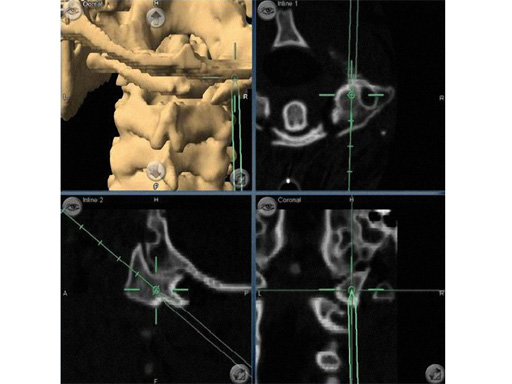
The most basic imaging for spinal surgery consists of static AP and lateral x-rays or image intensification during surgery. In CAS a virtual representation of the surgeons instruments are shown in relation to the patients anatomy that is displayed on a separate computer screen. CT scans or image intensifier images are used to generate the virtual surgical reality. This surgical GPS requires the attachment of a reference array with reflective beads to the patients spinal anatomy and to the surgical instrument to be tracked. The 2-D information obtained by two infrared cameras tracking these beads is converted into a 3-D representation based on the different reflective angles (Fig 1). Tracking using electromagnetic instead of infrared technology is currently being evaluated and has shown promising results [6, 7].
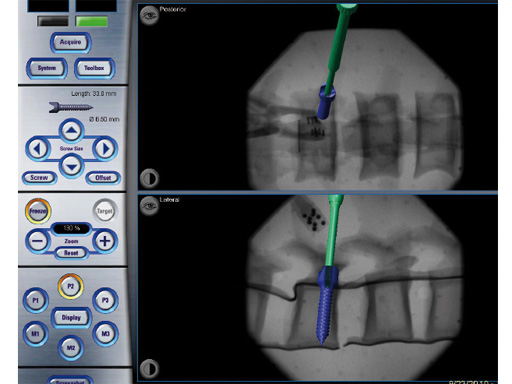
Fig 2 2-D navigation uses image-intensification-based AP and lateral images to track an instruments position in relation to spinal anatomy.
2-D navigation (2-D-nav) uses image-intensification-based AP and lateral images to track an instruments position in relation to the spinal anatomy. It is easier to set up but provides only 2-D information (Fig 2).
3-D navigation (3-D-nav) uses preoperative or intraoperative CTlike scans.
Preoperative CT scans require matching the patients bony anatomy with the scan which typically requires surgical exposure of certain landmarks. Alternatively, intraoperative AP and lateral image intensification can be used to match the preoperative CT scan with the actual patients anatomy in the operating room.
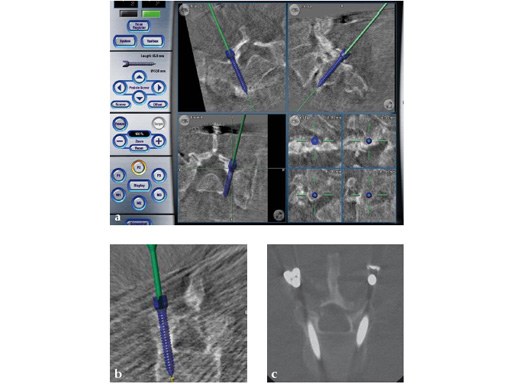
Intraoperative CT scans can be obtained using image-intensification- based isocentric C-arms, portable cone beam CT (O-arm, Medtronic) or true intraoperative CT scanners (iCT). Isocentric C-arms and portable scanners offer the advantage that they can also be used as regular C-arms, however their imaging quality may be inferior to stationary CT scans (Fig 3).
Fig 3ac a Intraoperative CT scans from an image-intensification-based isocentric C-arm. The software allows simulation of various diameter and length screws. bc Comparison of intraoperative simulation and postoperative CT scan shows the high accuracy of CAS.
Potential advantages and disadvantages of CAS
Supporters of CAS state that stereotactic navigation has the potential to:
- Improve accuracy of instrumentation placement and optimize the size of instrumentation used
- Reduce radiation exposure to surgeon and staff
- Enable less-invasive approaches through smaller access
- Allow preoperative planning of instrumentation size and trajectories and osteotomy procedures
- Allow verification of screw accuracy intraoperatively (intraoperative scanners only)
- Minimize the risks of wrong-level surgery
- Decrease reoperation rate
Potential disadvantages of CAS include:
- The learning curve associated with the technologies for the surgeon and the OR staff could be significant
- Upfront costs of the capital equipment
- Interruption of surgical flow
- Additional equipment and footprint in the OR
- Limited imaging quality and field of view with mobile 3-D imaging devices currently on the market.
- Potential increase in OR time
- Potential line-of-sight limitations for optical systems
- Concerns about accuracy and interference with metallic instruments using electromagnetic navigation systems
Application within spinal surgery
Within spinal surgery CAS is typically used for placement of instrumentation. A recent survey among AOSpine surgeons (Hrtl, et al, unpublished 2010) showed that CAS is particularly helpful in three areas: complex spinal surgery, such as deformity and revision surgery; minimally invasive spinal surgery (MIS); and surgery in challenging anatomy, such as thoracic and cervical instrumentation.
Deformity and revision surgery
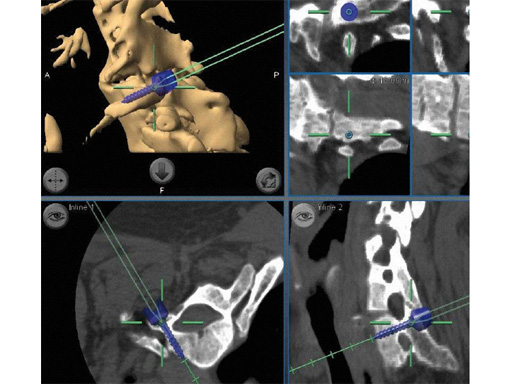
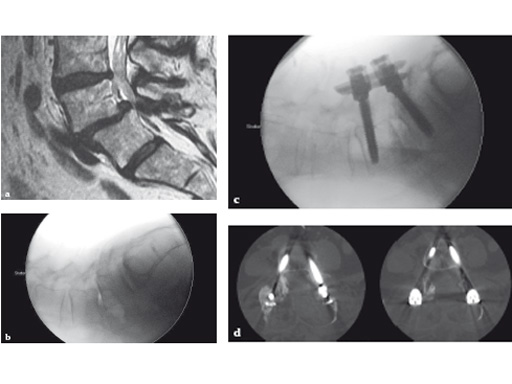
Placement of instrumentation in anatomy that has been distorted due to significant deformity or due to previous surgery and subsequent scarring and ossification can be difficult and is associated with an increased rate of misplacement. In these cases CAS allows precise placement of instrumentation even in the absence of regular anatomical landmarks (Fig 4).
Fig 4 In this cervical deformity case extensive ossification was present and regular landmarks not available. Navigation allowed precise placement of cervical pedicle screws.
Recently, CAS has also been advocated for virtual surgical planning of osteotomy procedures and for intraoperative navigation in adult deformity correction surgery [8].
Minimally invasive spinal surgery (MIS)
The goal of MIS is to achieve outcomes that are comparable or superior to conventional surgery but with less postoperative pain, quicker recovery, reduced blood loss, less soft-tissue damage, smaller surgical incisions and less scarring. The concept of MIS evolved out of the advancements achieved in four different surgical fields over the past years:
- Microsurgery using the microscope or endoscope
- New spinal access strategies via percutaneous or miniopen procedures
- Neuronavigation using 2- or 3-D imaging technology
- New instrumentation
Two recent clinical studies demonstrated improved screw accuracy with isocentric image-intensification-based CT navigation compared to conventional image intensification in > 300 patients undergoing minimally invasive lumbar fusion [9, 10]. Stereotactic navigation is especially useful in patients with more complex anatomy, such as significant spondylolisthesis or degenerative scoliosis. For example, the combination of microsurgery, tubular access approach, CAS and modern instrumentation technology allow decompression and reduction of lumbar spondylolisthesis and stenosis with minimal blood loss and injury to the surrounding musculature (Fig 5). Navigation can also be used to determine the best trajectory for intervertebral cage placement and for transsacral fixation [11]. In the lumbar spine it can be used to determine the length of rods and to align screws during a multilevel fusion so that the percutaneous rod placement is facilitated (Fig 6). Stereotactic navigation has also enabled the minimally invasive resection of odontoid masses via a transnasal route, which is a significant improvement when compared to conventional maximally invasive transoral surgery [12, 13] (Fig 7).
Fig 5ad a Preoperative MRI scan: significant spondylolisthesis in a symptomatic patient.
b Intraoperative x-ray of same patient:
progression to grade II spondylolisthesis in prone position on OR table.
c Minimally invasive surgery allowed decompression, discectomy, instrumentation, and reduction in this patient through two small skin incisions with minimal blood loss.
d Postoperative CT scan: accurate positioning of instrumentation. 3-D-nav allowed for optimization of screw length and diameter given the patients anatomy.
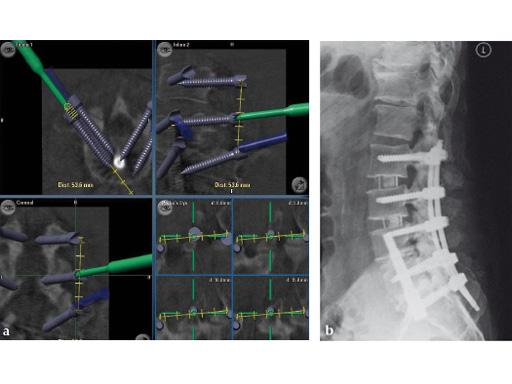
Fig 6ab a Intraoperative screenshot of multilevel lumbar instrumentation. By simulating multiple screws a screw angle and trajectory can be chosen that will facilitate smooth insertion of rods. Distance between screws and rod length can also be determined.
b Postoperative lateral x-ray in same patient. This case combined a lateral translumbar approach for interbody fusion L2L4, a transsacral discectomy, instrumentation, and fusion for L4S1 and percutaneous pedicle screw instrumentation from L2S1 using 3-D-nav.
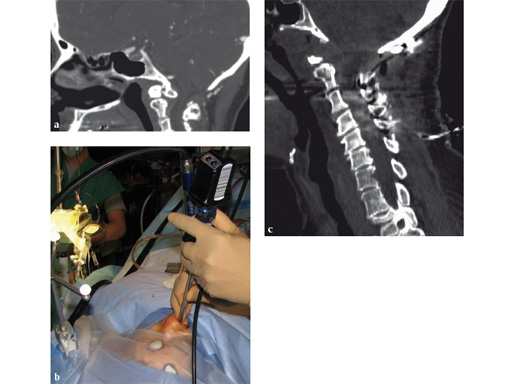
Fig 7ac a Preoperative CT scan: os odontoideum with compression of cervicomedullary junction in a symptomatic patient.
b Intraoperative view of navigated endoscope entering nose. Reference array for navigation has been attached to skull clamp.
c Postoperative CT scan: complete resection of the mass. Patient also underwent occipitocervical instrumentation and fusion.
Special anatomy: cervical and thoracic instrumentation
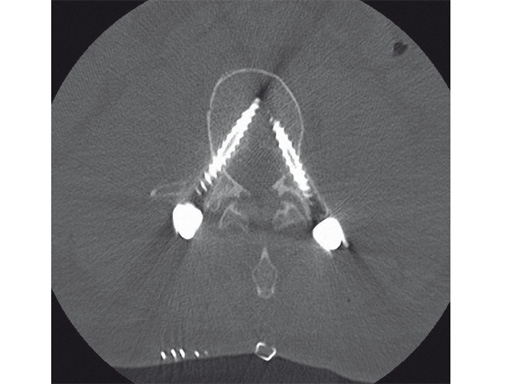
Instrumentation of the cervical and thoracic spine can entail special challenges, even with open surgery and relatively straightforward anatomy. Screw placement with CAS can facilitate the placement of pedicle screws with very high precision (Figs 4, 8). A recent publication has demonstrated that for thoracic pedicle screws the use of CAS resulted in reduction in operative time and improvement in screw accuracy [14]. In the cervical spine placement of pedicle screws in patients has been accomplished with higher accuracy using 3-D-nav when compared to conventional techniques [15]. In the high cervical spine it can be used for accurate instrumentation of the occiput, C1 and C2 (Fig 9) [16].
AO survey on use of navigation in spine surgery
Despite reports suggesting that CAS can improve accuracy of screw placement and decrease radiation exposure [1, 2, 14, 1721], it has not been generally accepted among spine surgeons. The reasons for this are complex and may involve factors related to availability, training, individual experience and preference, economical factors, and many more.
A better understanding of these reasons would be essential in order to determine the current deficits of navigation and how these can be addressed. Therefore, the previous Access and Navigation Expert Group decided to perform an internet-based survey among AOSpine surgeons in order to better understand current attitudes towards spinal navigation (Hrtl, et al, unpublished 2010).
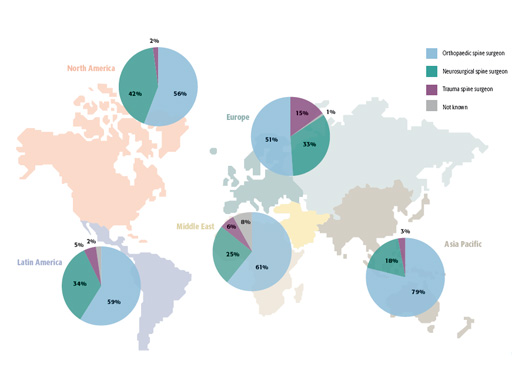
678 out of 3,348 contacted spine surgeons (20%) responded to a 12-item questionnaire that assessed the experiences with CAS and their current attitudes towards its use in spine surgery (Fig 10). The results showed that while 35% of overall surgeons actually had CAS available at their institution only few used CAS routinely. Despite an even more widespread distribution of navigation systems in North America and Europe only 11% used it routinely. Surgeons performing a high volume of spinal fusions are more likely to use CAS than surgeons performing a lower volume of spinal fusions, indicating that high-volume users may have integrated CAS more effectively into their workflow. This goes against previous thoughts that navigation may be used by surgeons who are not that familiar with instrumentation and would rely on CAS to facilitate procedures in the OR. Routine users considered its accuracy, the potential of facilitating complex surgery, and the reduction in radiation exposure as the main advantages of CAS. Routine users identified the increase in OR time associated with CAS as the main reason why they did not use CAS more frequently. The lack of equipment, inadequate training, and high costs were quoted by the majority of the nonusers as the main reasons for not using CAS. The survey also showed that 42% of surgeons felt positively about CAS and that 37% had strong and very strong positive beliefs about the benefits of navigation. Overall, neurological surgeons and surgeons with a busy MIS practice were more likely to use CAS. In summary, the survey demonstrated that the majority of responding surgeons had positive beliefs about navigation.
However, the high costs associated with CAS systems, lack of access to navigation systems, the increase in OR time, and the lack of hard scientific data supporting its clinical benefits, appear to be the main reasons why surgeons do not use CAS or do not use it more frequently.
Conclusion
The integration of CAS into spinal surgery holds great promise and (class III) clinical evidence supports its benefits especially in complex surgery, minimally invasive procedures, and for instrumentation of challenging anatomy, such as in the thoracic and cervical spine. Better designed studies and trials are needed to confirm these benefits. Overall, surgeons have positive opinions about CAS but the current technology needs to be improved in order to make CAS more user friendly and time efficient. In order to reach more surgeons the technology has to be affordable and better training opportunities must be offered. The future of CAS will include more widespread access to better imaging technologies, such as intraoperative CT scanning and the combination of CAS with different imaging modalities and possibly intraoperative functional monitoring, such as electrophysiology [22].
References
1 Nolte LP, Zamorano L, Arm E, et al (1996) Image-guided computer-assisted spine surgery: a pilot study on pedicle screw fixation. Stereotact Funct Neurosurg; 66(13):108117.
2 Nolte LP, Zamorano L, Visarius H, et al (1995) Clinical evaluation of a system for precision enhancement in spine surgery. Clin Biomech; 10(6):293303.
3 Foley KT, Smith MM (1996) Image-guided spine surgery. Neurosurg Clin N Am; 7(2):171186.
4 Kalfas IH, Kormos DW, Murphy MA, et al (1995) Application of frameless stereotaxy to pedicle screw fixation of the spine. J Neurosurg; 83(4):641677.
5 Langoltz F, Nolte LP (2007) Computer-assisted surgery. Aebi M, Arlet V, Webb JK (eds), AOSpine Manual, Principles and Techniques. Stuttgart New York:Thieme, 573587.
6 Fraser JF, von Jako R, Carrino JA, et al (2008) Electromagnetic navigation in minimally invasive spine surgery: results of a cadaveric study to evaluate percutaneous pedicle screw insertion. SAS Journal; 2:4347.
7 von Jako RA, Carrino JA, Yonemura KS, et al (2009) Electromagnetic navigation for percutaneous guide-wire insertion: accuracy and efficiency compared to conventional fluoroscopic guidance. Neuroimage; 47 Suppl 2:T127132.
8 Metz LN, Burch S (2008) Computer-assisted surgical planning and image-guided surgical navigation in refractory adult scoliosis surgery: case report and review of the literature. Spine; 33(9):E287292.
9 Nakashima H, Sato K, Ando T, et al (2009) Comparison of the percutaneous screw placement precision of isocentric C-arm 3-dimensional fluoroscopy-navigated pedicle screw implantation and conventional fluoroscopy method with minimally invasive surgery. J Spinal Disord Tech; 22(7):468472.
10 Fraser J, Gebhard H, Irie D, et al (2010) Iso-C/3 dimensional neuronavigation versus conventional fluoroscopy for minimally invasive Pedicle Screw Placement in Lumbar Fusion; Minim Invas Neurosurg; in press.
11 Luther N, Tomasino A, Parikh K, et al (2009) Neuronavigation in the minimally invasive presacral approach for lumbosacral fusion. Minim Invas Neurosurg; 52(4):196200.
12 Leng LZ, Anand VK, Hrtl R, et al (2009) Endonasal endoscopic resection of an os odontoideum to decompress the cervicomedullary junction. Spine; 34(4):E139E143.
13 Laufer I, Greenfield JP, Anand VK, et al (2008) Endonasal endoscopic resection of the odontoid process in a nonachondroplastic dwarf with juvenile rheumatoid arthritis: feasibility of the approach and utility of the intraoperative iso-C threedimensional navigation. J Neurosurg Spine; 8(4):376380.
14 Rajasekaran S, Vidyahara S, Ramesh P, et al (2007) Randomized clinical study to compare the accuracy of navigated and non-navigated thoracic pedicle screws in deformity correction surgeries. Spine; 32(2):E5664.
15 Richter M, Cakir B, Schmidt R (2005) Cervical pedicle screws: conventional versus computer-assisted placement of cannulated screws. Spine; 30(20):22802287.
16 Nottmeier EW, Young PM (2010) Image-guided placement of occipitocervical instrumentation using a reference arc attached to the headholder. Neurosurgery; 66(3 Suppl Operative):138142.
17 Nottmeier, EW, Seemer W, Young PM (2009) Placement of thoracolumbar pedicle screws using three-dimensional image guidance: experience in a large patient cohort. J Neurosurg Spine; 10(1):3339.
18 Berlemann U, Monin D, Arm E, et al (1997) Planning and insertion of pedicle screws with computer assistance. J Spinal Disord; 10(2):117124.
19 Laine T, Schlenzka D, Makitalo K, et al (1997) Improved accuracy of pedicle screw insertion with computer-assisted surgery. A prospective clinical trial of 30 patients. Spine; 22(11):12541258.
20 Schwarzenbach O, Berlemann U, Jost B, et al (1997) Accuracy of computerassisted pedicle screw placement. An in vivo computed tomography analysis. Spine; 22(4):452458.
21 Merloz P, Tonetti J, Pittet L, et al (1998) Pedicle screw placement using image guided techniques. Clin Orthop Relat Res; 354:3948.
22 Idler C, Rolfe K, Gorek JE (2010) Accuracy of percutaneous lumbar pedicle screw placement using the oblique or owls-eye view and novel guidance technology. J Neurosurg Spine; 509515.
Hazards and labeling
Due to varying countries’ legal and regulatory approval requirements, consult the appropriate local product labeling for approved intended use of the products described on this website. All devices on this website are approved by the AO Technical Commission. For logistical reasons, these devices may not be available in all countries worldwide at the date of publication.
Legal restrictions
This work was produced by AO Foundation, Switzerland. All rights reserved by AO Foundation. This publication, including all parts thereof, is legally protected by copyright.
Any use, exploitation or commercialization outside the narrow limits set forth by copyright legislation and the restrictions on use laid out below, without the publisher‘s consent, is illegal and liable to prosecution. This applies in particular to photostat reproduction, copying, scanning or duplication of any kind, translation, preparation of microfilms, electronic data processing, and storage such as making this publication available on Intranet or Internet.
Some of the products, names, instruments, treatments, logos, designs, etc referred to in this publication are also protected by patents, trademarks or by other intellectual property protection laws (eg, “AO” and the AO logo are subject to trademark applications/registrations) even though specific reference to this fact is not always made in the text. Therefore, the appearance of a name, instrument, etc without designation as proprietary is not to be construed as a representation by the publisher that it is in the public domain.
Restrictions on use: The rightful owner of an authorized copy of this work may use it for educational and research purposes only. Single images or illustrations may be copied for research or educational purposes only. The images or illustrations may not be altered in any way and need to carry the following statement of origin “Copyright by AO Foundation, Switzerland”.
Check www.aofoundation.org/disclaimer for more information.
If you have any comments or questions on the articles or the new devices, please do not hesitate to contact us.
“approved by AO Technical Commission” and “approved by AO”
The brands and labels “approved by AO Technical Commission” and “approved by AO”, particularly "AO" and the AO logo, are AO Foundation's intellectual property and subject to trademark applications and registrations, respectively. The use of these brands and labels is regulated by licensing agreements between AO Foundation and the producers of innovation products obliged to use such labels to declare the products as AO Technical Commission or AO Foundation approved solutions. Any unauthorized or inadequate use of these trademarks may be subject to legal action.
AO ITC Innovations Magazine
Find all issues of the AO ITC Innovations Magazine for download here.
Innovation Awards
Recognizing outstanding achievements in development and fostering excellence in surgical innovation.