3.5 Locking Attachment Plate
Michael Wagner
The 3.5 Locking Attachment Plate is indicated for use with locking compression plates (LCPs) to augment the stabilization of fractures, including periprosthetic fractures and fractures in the presence of intramedullary implants in the femur, tibia, and humerus, particularly in osteopenic bone.
Clinical problem
Periprosthetic fractures are increasing because of the aging of the population, more active old people, the development of endoprosthetics and an increase in the number of patients with long-standing implants. The incidence has been reported to be 1.5% for primary procedures and 4% for revisions [1]. Because of the prosthesis stem (or a nail) in the intramedullary canal it is not possible to insert screws bicortically as it would be needed. These fractures are mainly treated with cable systems and monocortical screws with the disadvantage of high invasiveness and the possibility of cutting into osteoporotic bone.
Solution
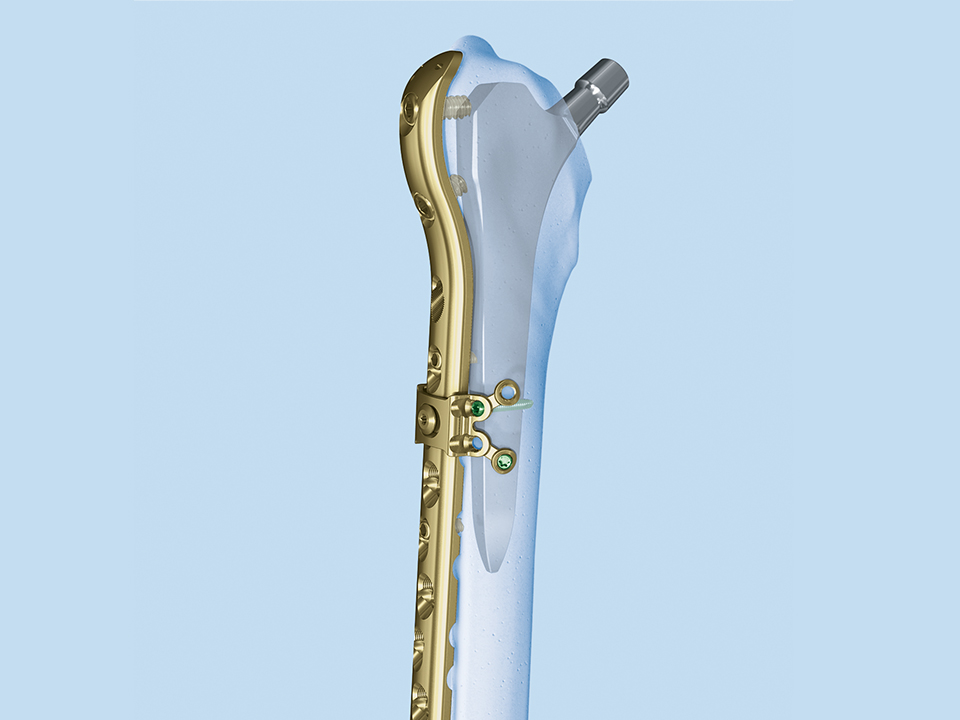
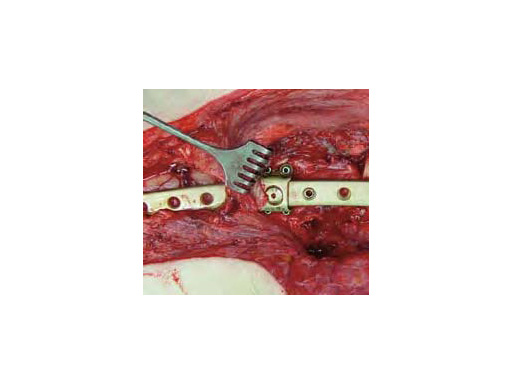
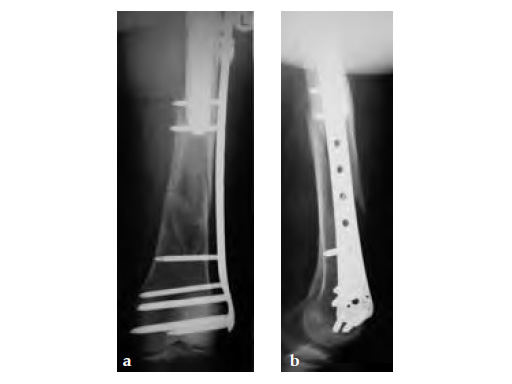
The 3.5 Locking Attachment Plate which can be attached to an LCP offers an alternative to cables (Fig 1). With the possibility to insert 3.5 mm locking screws bicortically by avoiding the prosthesis stem (Fig 2) a similar or even better mechanical stability than with cables can be reached with a less invasive procedure. The plate is attached to a base plate, in essence, widening that plate and its screw angle options to gain 3.5 mm screw purchase around a prosthesis or any device that may be blocking the intramedullary canal.
If necessary the plate can be adapted onto the bone shape. If the bone diameter is small, the plate can be bent, and screw angulations increased for the screws to get hold in the cortex.
The plate is simple to use and the decision can be made intraoperatively (implants are available, both nonsterile and sterile packed). The threaded insert portion of the connecting screw is inserted into the base plate. Then the plate is placed on top and locked with the upper portion of the connecting screw.
The 3.5 Locking Attachment Plate can also be used to prevent lateral screw pull-out in osteoporotic bone irrespective of a prosthesis or an intramedullary implant.
References
Old AB, McGrory BJ, White RR, et al Fixation of Vancouver B1 Periprosthetic fractures by broad metal plates without the application of strut allografts. J Bone Joint Surg Br. 2006; 88(11): 1425-1429.
Case provided by Klaus-Dieter Schaser, Berlin, Germany
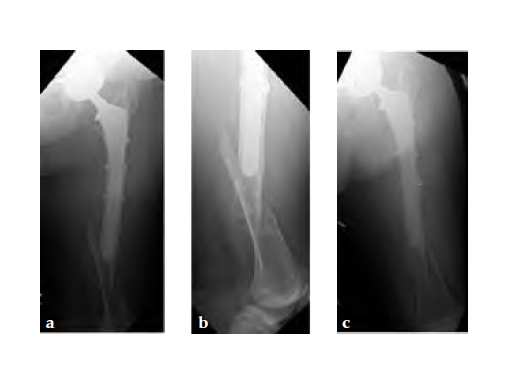
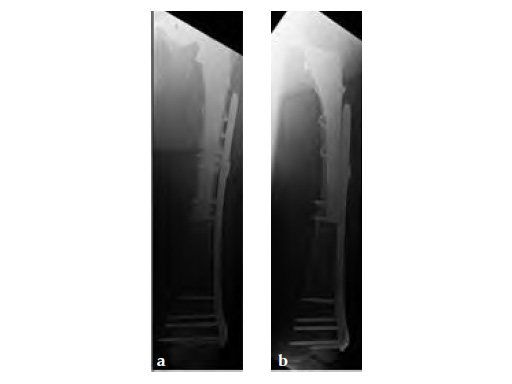
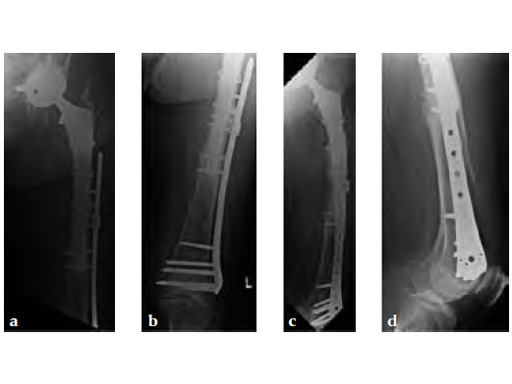
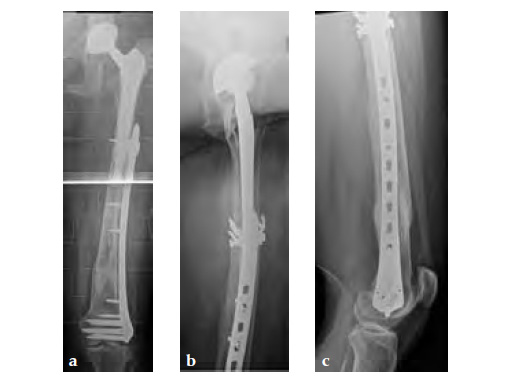
Case 1: A 78-year-old female sustained a periprosthetic fracture, Vancouver type C, 9 years after a total hip arthroplasty.
Case provided by Michael Wagner, Wien, Austria.
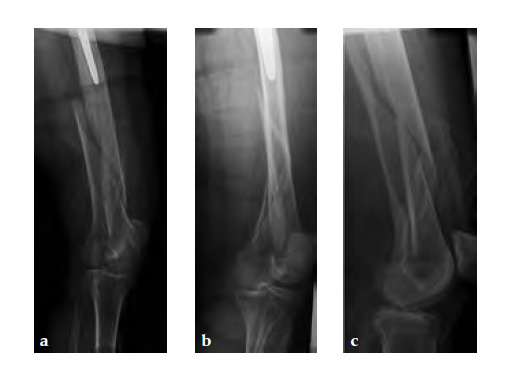
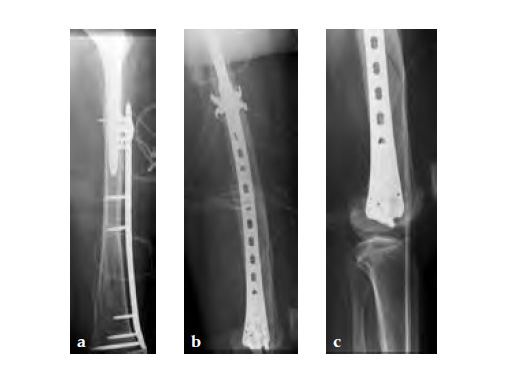
Case 2: A 76-year-old female with a Vancouver type C fracture.
Hazards and labeling
Due to varying countries’ legal and regulatory approval requirements, consult the appropriate local product labeling for approved intended use of the products described on this website. All devices on this website are approved by the AO Technical Commission. For logistical reasons, these devices may not be available in all countries worldwide at the date of publication.
Legal restrictions
This work was produced by AO Foundation, Switzerland. All rights reserved by AO Foundation. This publication, including all parts thereof, is legally protected by copyright.
Any use, exploitation or commercialization outside the narrow limits set forth by copyright legislation and the restrictions on use laid out below, without the publisher‘s consent, is illegal and liable to prosecution. This applies in particular to photostat reproduction, copying, scanning or duplication of any kind, translation, preparation of microfilms, electronic data processing, and storage such as making this publication available on Intranet or Internet.
Some of the products, names, instruments, treatments, logos, designs, etc referred to in this publication are also protected by patents, trademarks or by other intellectual property protection laws (eg, “AO” and the AO logo are subject to trademark applications/registrations) even though specific reference to this fact is not always made in the text. Therefore, the appearance of a name, instrument, etc without designation as proprietary is not to be construed as a representation by the publisher that it is in the public domain.
Restrictions on use: The rightful owner of an authorized copy of this work may use it for educational and research purposes only. Single images or illustrations may be copied for research or educational purposes only. The images or illustrations may not be altered in any way and need to carry the following statement of origin “Copyright by AO Foundation, Switzerland”.
Check www.aofoundation.org/disclaimer for more information.
If you have any comments or questions on the articles or the new devices, please do not hesitate to contact us.
“approved by AO Technical Commission” and “approved by AO”
The brands and labels “approved by AO Technical Commission” and “approved by AO”, particularly "AO" and the AO logo, are AO Foundation's intellectual property and subject to trademark applications and registrations, respectively. The use of these brands and labels is regulated by licensing agreements between AO Foundation and the producers of innovation products obliged to use such labels to declare the products as AO Technical Commission or AO Foundation approved solutions. Any unauthorized or inadequate use of these trademarks may be subject to legal action.
AO ITC Innovations Magazine
Find all issues of the AO ITC Innovations Magazine for download here.
Innovation Awards
Recognizing outstanding achievements in development and fostering excellence in surgical innovation.