Variable Angle LCP Distal Radius System 2.4
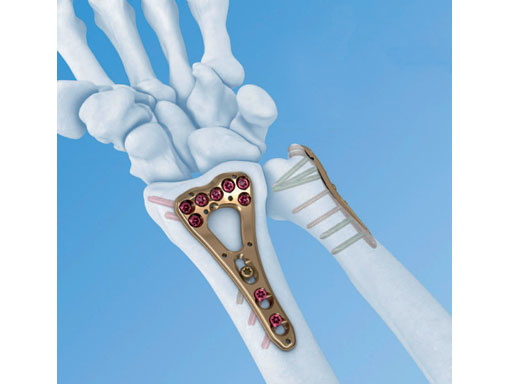
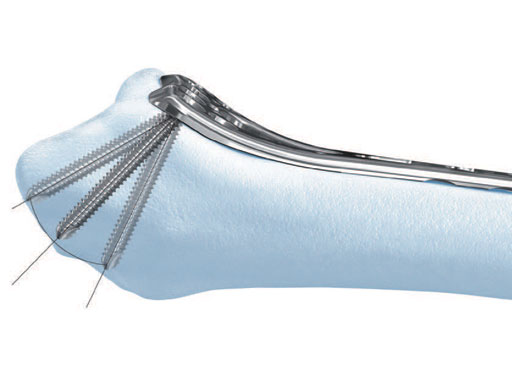
The variable angle LCP distal radius system 2.4 is indicated for fixation of complex intra- and extraarticular fractures and osteotomies of the distal radius and other small bones (Fig 1). The new variable angle technology enhances fragment-specific fracture fixation by providing the flexibility to lock screws in trajectories that diverge up to 15 from the central axis of the plate hole (Fig 2). The plates are precontoured to match the anatomy of the volar distal radius. Variable angle locking holes in the head of the plate enable placement of the screws at the most appropriate position to create a locking construct to support the articular surface and reduce the need for bone graft.
The system consists of the variable angle LCP two-column volar distal radius 2.4 and the variable angle LCP volar extraarticular distal radius 2.4. Both plate types use 2.4/2.7 mm cortex or 2.4 mm locking screws in the plate shaft, and 2.4 mm variable angle locking screws in the head of the plate. The variable angle locking screws are available in sizes from 8 mm to 30 mm. All plates are available in stainless steel or CP4 titanium.
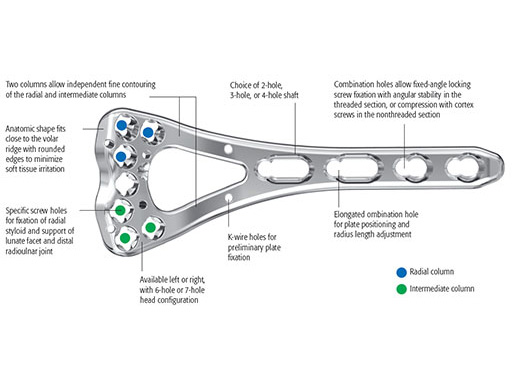
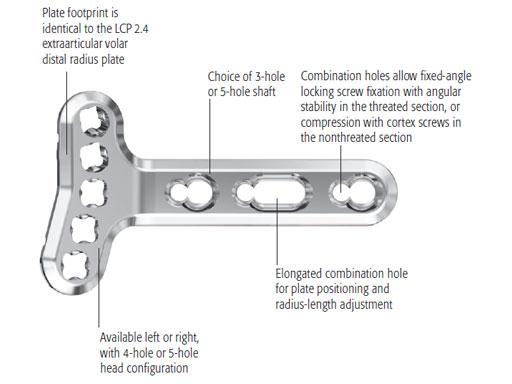
Variable Angle LCP Two-Column Volar Distal Radius 2.4
The plates come in a left and right version. The plate head has a 6-hole and a 7-hole head option, the shaft features 2-, 3-, and 4-hole versions. In total, twelve different plates are available (see also case below).
VA LCP Two-Column Plate-Extra Long
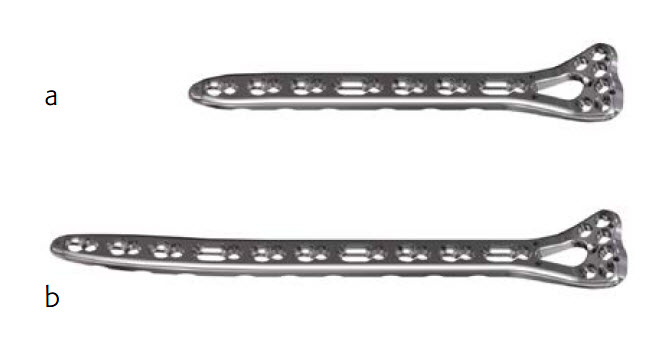
In December 2016, AOTK Trauma approved the VA LCP Two-Column Extra Long Plate designed for the treatment of intra- and extraarticular fractures, osteotomies, and nonunions and malunions of the distal radius. The plate is available with 7, 10, and 13 holes in the shaft and is indicated for fixation with or without extension into the radial diaphysis. Use of trial implants can assist with determining the plate size appropriate for the patient.
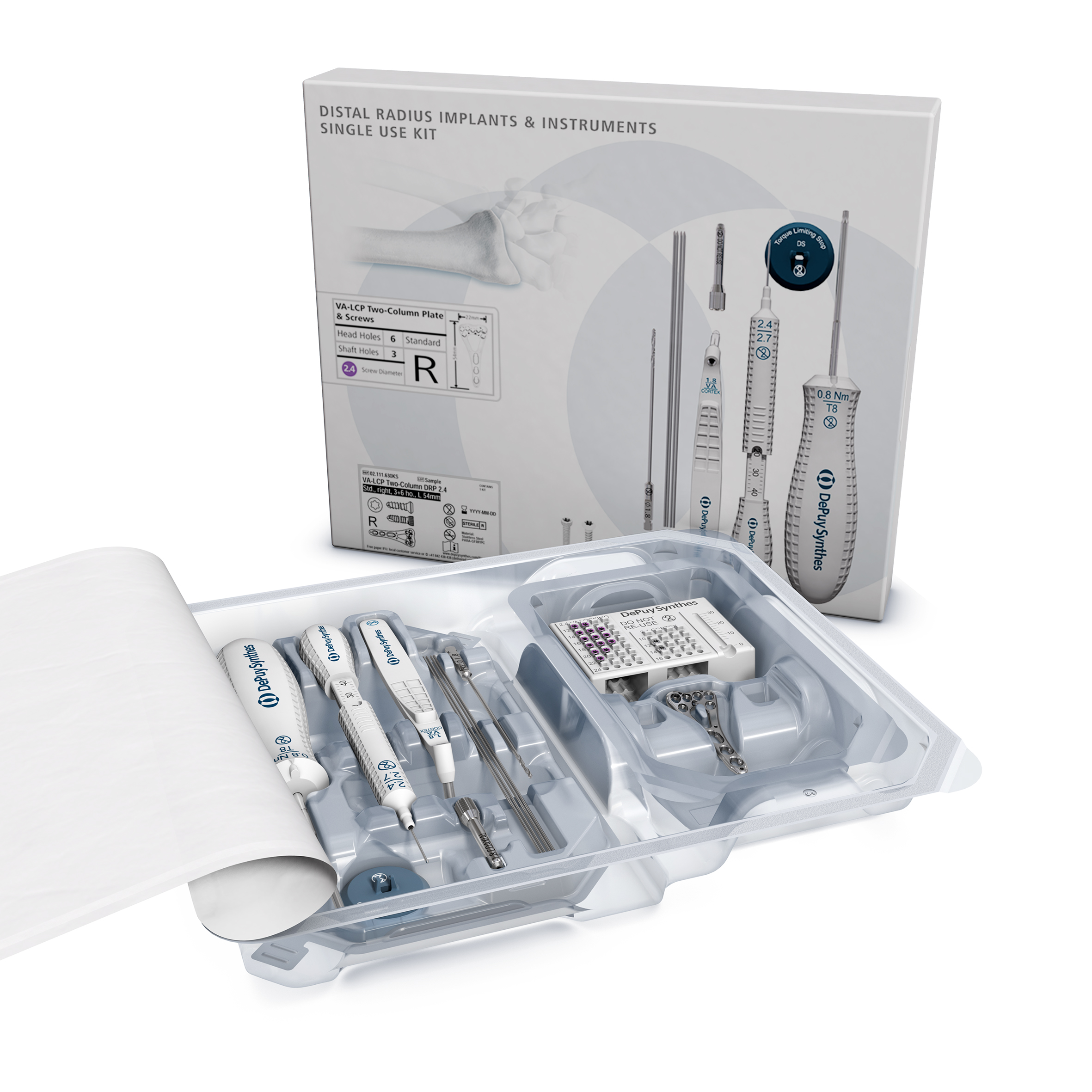
Distal Radius Sterile Kit
Following the successful release of the VA Two-Column Plate in 2009, this anatomical palmar implant is now available as part of a new distal radius sterile kit (Fig 1) recently approved by the AOTK. The creation of a streamlined modular system represents the optimization of workflow efficiency in the operating room and drives repeatable consistent procedures by providing a complete core kit. The prepackaged implants and single-use instruments eliminate the need for sterilization and its subsequent operational cost. The disposable instruments are designed to a high standard, which enables the user to maintain the same surgical technique performed with conventional reusable instruments (Fig 2).
Variable Angle LCP Volar Extraarticular Distal Radius 2.4
The new plate design is based on the existing LCP volar extraarticular distal radius 2.4. In total, eight plates are available, left and right, either 4-hole or 5-hole head option, and 3- and 5-hole shaft versions (see also case below).
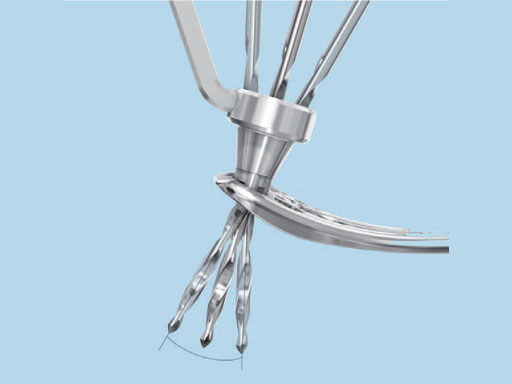
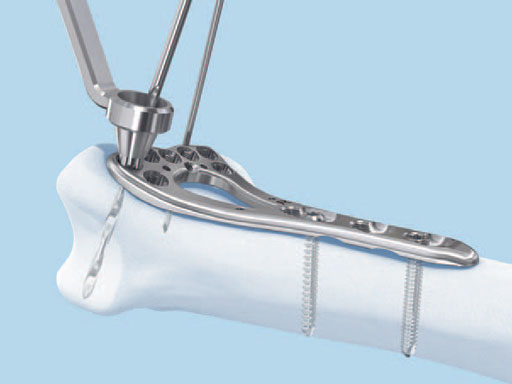
1.8 mm VA Locking Buttress Pins
The 1.8 mm variable angle (VA) locking buttress pins are used for fixation of complex intra- and extraarticular fractures and osteotomies of the distal radius and other small bones. They are part of the VA-LCP distal radius system.
The VA locking head with T8 StarDrive recess allows the pin to lock up to 15 off-axis in any variable angle locking hole, or on-axis in any locking hole to provide angular stability at angles determined by the surgeon. The 1.8 mm diameter shaft is smooth and nonthreaded with a blunt, rounded tip which enables easy insertion and provides buttressing for the subchondral bone of complex and comminuted fractures. The rounded tip lowers tendon irritation when penetrating the far cortex. It also draws the surgeons attention to the principles of angular stability, where it is unnecessary for a locking screw or buttress pin to breach the far cortex at all. Leaving the tip of a locking screw or buttress pin just underneath the far cortex provides equal stability to engaging the far cortex with screw threads, particularly in osteopenic bone, thereby removing any risk of tendon irritation or injury. 4 mm of neck threads in the shaft aid in backing out the pin for easy removal.
The pins are available in titanium and stainless steel in lengths of 830 mm.
A special drill guide allows up to a 15 angulation around the central axis of the locking hole (Fig 3).
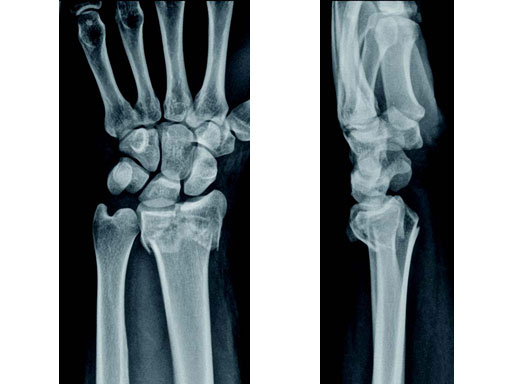
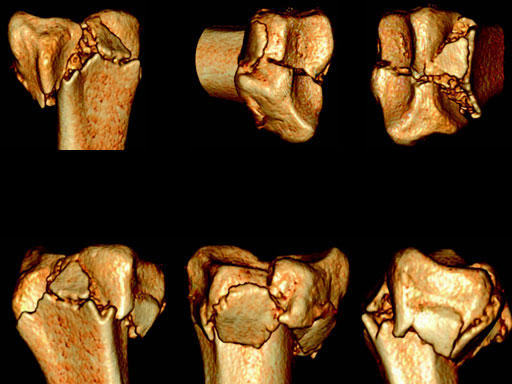
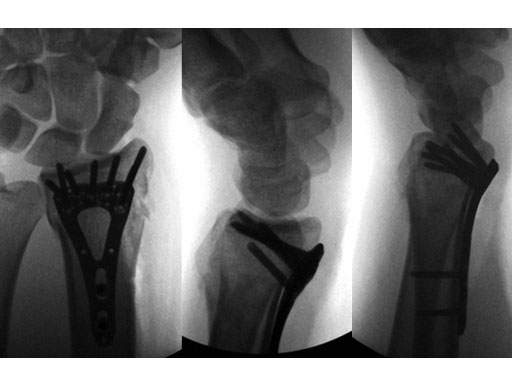
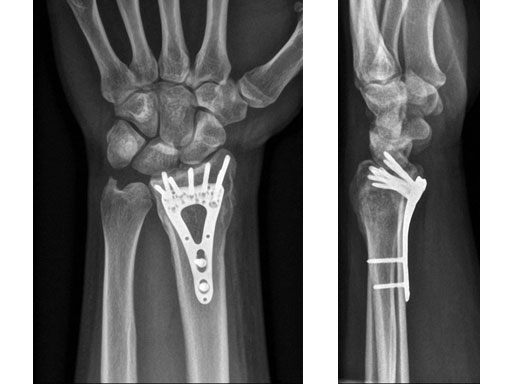
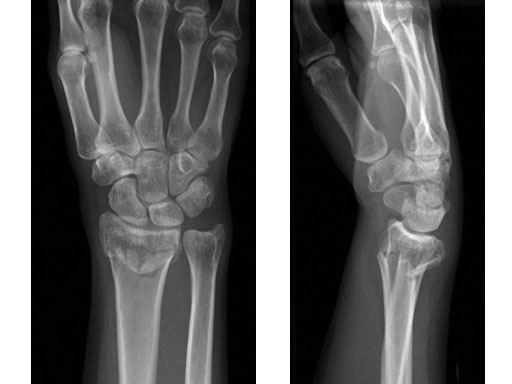
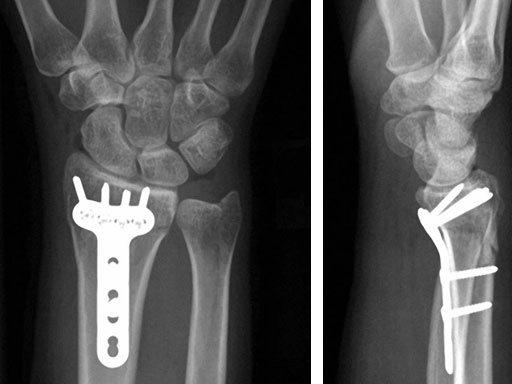
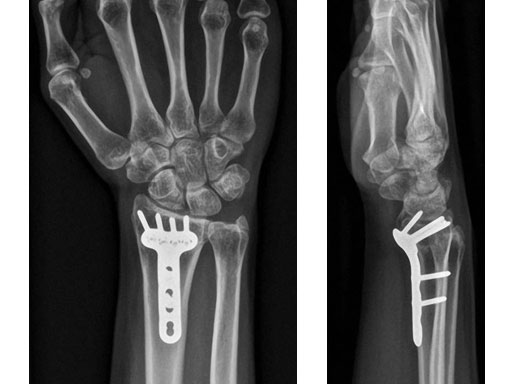
Case 1: 22-year-old male fell off a horse while playing polo.
Case provided by Ladislav Nagy, Zrich, CH
Fig 1a-b Preoperative x-rays.
Fig 2af Preoperative 3-D CT scans.
Fig 3ac Immediate postoperative x-rays.
Fig 4ab X-rays 3 months postoperatively.
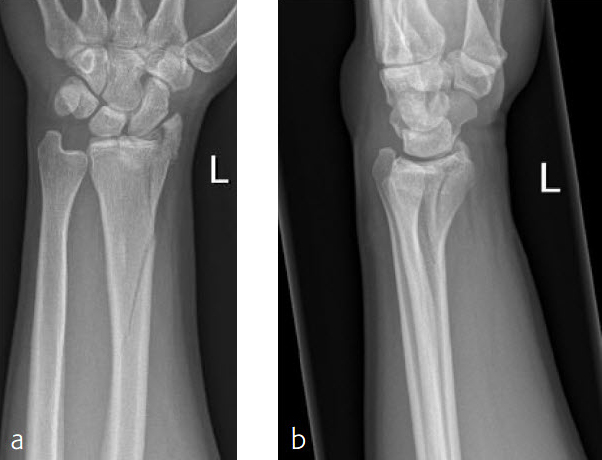
Case 2: 36-year-old female fell down on a meadow.
Case provided by Ladislav Nagy, Zrich, CH
Fig 1ab Preoperative x-rays.
Fig 2ab X-rays postoperatively.
Fig 3ab X-rays 5 months postoperatively.
Cases 3 and 4 provided by Max Daniel Kauther and Marcel Dudda, Essen, Germany
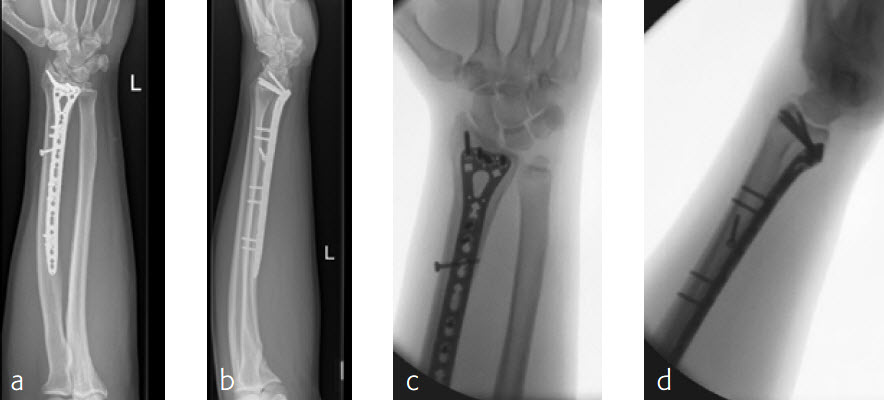
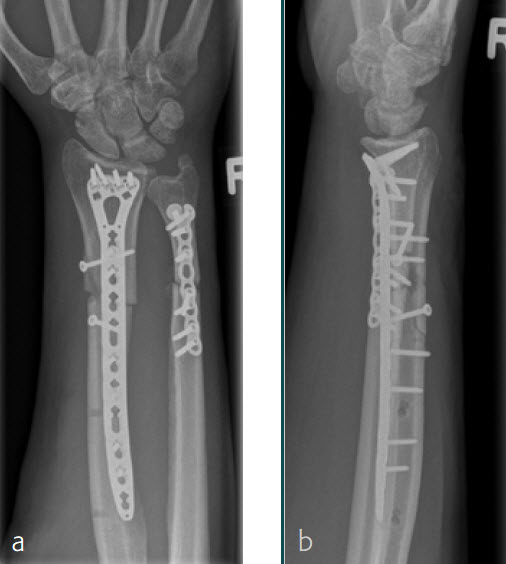
Case 3: Multifragmentary distal radius fracture with extension into the diaphysis
A 26-year-old man suffered a multifragmentary fracture of his left distal radius with extension into the diaphysis (AO23 C3.3) (Fig 1). The VA LCP Extra Long Two-Column plate was used for fixation (Fig 2). After initial immobilization, the plate provided a good postoperative fixation of the fracture.
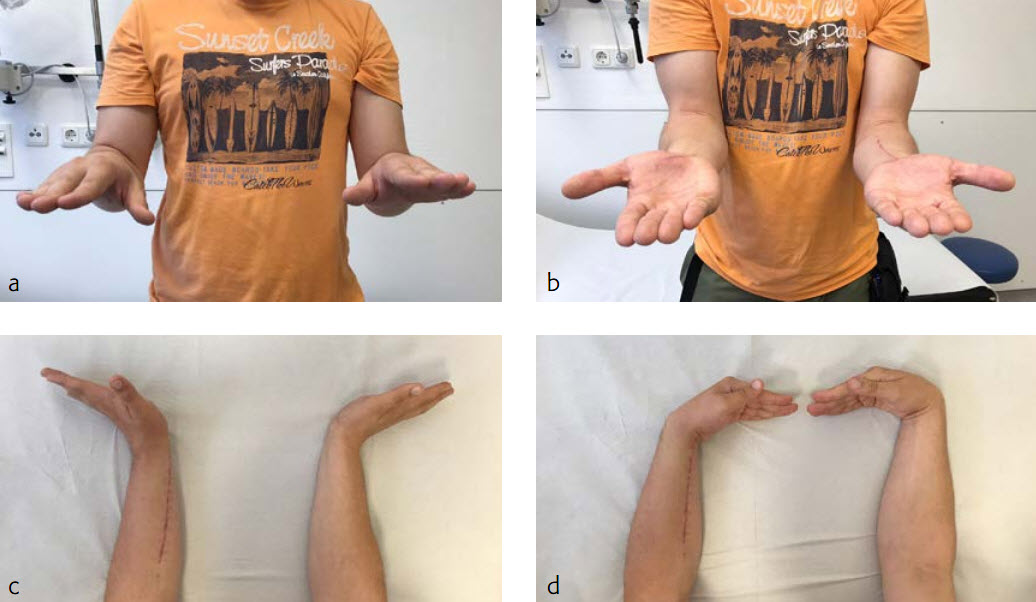
The fracture showed primary bone healing without callus formation. At the 3-month follow-up, the patient was full weight bearing with excellent clinical function (Fig 3). The radiological follow-up can be technically challenging due to the correct focus of the central ray.
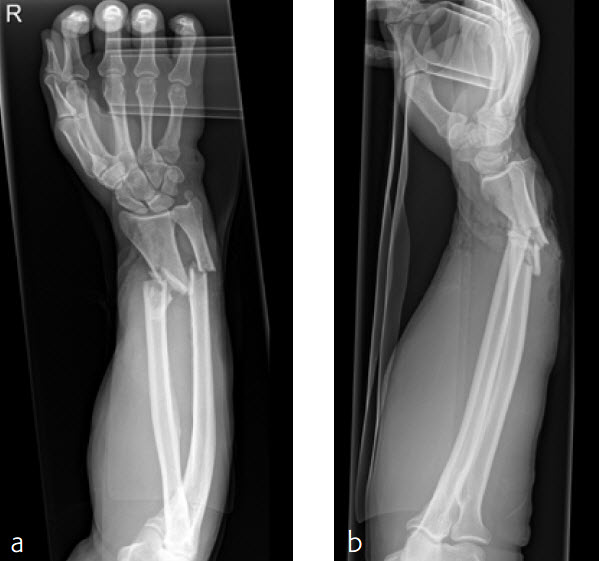
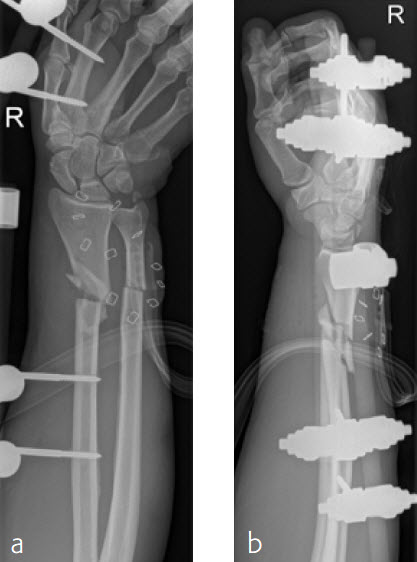
Case 4: Open radius shaft fracture
A 59-year-old farmer suffered a crush injury with an open forearm fracture (AO22 C2, Gustilo and Anderson IIIB) (Fig 1). Initial stabilization was carried out by external fixator (Fig 2). After four rounds of debridement and capillary ingrowth of a splitting skin graft at day 17, the VA LCP Extra Long Two-Column plate was used for fixation of the radius. A 2.7 mm LCP Condylar Plate was used for fixation of the ulna. The plates provided good stability for a functional after-treatment.
At the 3-month follow-up, the patient was full weight bearing with healing fractures (Fig 3).
Hazards and labeling
Due to varying countries’ legal and regulatory approval requirements, consult the appropriate local product labeling for approved intended use of the products described on this website. All devices on this website are approved by the AO Technical Commission. For logistical reasons, these devices may not be available in all countries worldwide at the date of publication.
Legal restrictions
This work was produced by AO Foundation, Switzerland. All rights reserved by AO Foundation. This publication, including all parts thereof, is legally protected by copyright.
Any use, exploitation or commercialization outside the narrow limits set forth by copyright legislation and the restrictions on use laid out below, without the publisher‘s consent, is illegal and liable to prosecution. This applies in particular to photostat reproduction, copying, scanning or duplication of any kind, translation, preparation of microfilms, electronic data processing, and storage such as making this publication available on Intranet or Internet.
Some of the products, names, instruments, treatments, logos, designs, etc referred to in this publication are also protected by patents, trademarks or by other intellectual property protection laws (eg, “AO” and the AO logo are subject to trademark applications/registrations) even though specific reference to this fact is not always made in the text. Therefore, the appearance of a name, instrument, etc without designation as proprietary is not to be construed as a representation by the publisher that it is in the public domain.
Restrictions on use: The rightful owner of an authorized copy of this work may use it for educational and research purposes only. Single images or illustrations may be copied for research or educational purposes only. The images or illustrations may not be altered in any way and need to carry the following statement of origin “Copyright by AO Foundation, Switzerland”.
Check www.aofoundation.org/disclaimer for more information.
If you have any comments or questions on the articles or the new devices, please do not hesitate to contact us.
“approved by AO Technical Commission” and “approved by AO”
The brands and labels “approved by AO Technical Commission” and “approved by AO”, particularly "AO" and the AO logo, are AO Foundation's intellectual property and subject to trademark applications and registrations, respectively. The use of these brands and labels is regulated by licensing agreements between AO Foundation and the producers of innovation products obliged to use such labels to declare the products as AO Technical Commission or AO Foundation approved solutions. Any unauthorized or inadequate use of these trademarks may be subject to legal action.
AO ITC Innovations Magazine
Find all issues of the AO ITC Innovations Magazine for download here.
Innovation Awards
Recognizing outstanding achievements in development and fostering excellence in surgical innovation.