LCP Wrist Fusion Plate
The LCP wrist fusion system is indicated for wrist arthrodesis. Specificindications include posttraumatic arthritis of the joints of the wrist, rheumatoid wrist deformities requiring restoration, complex carpal instability, sequelae of septic arthritis of the wrist, severe unremitting wrist pain related to motion, brachial plexus nerve palsies, tumor resection, and spastic deformities.
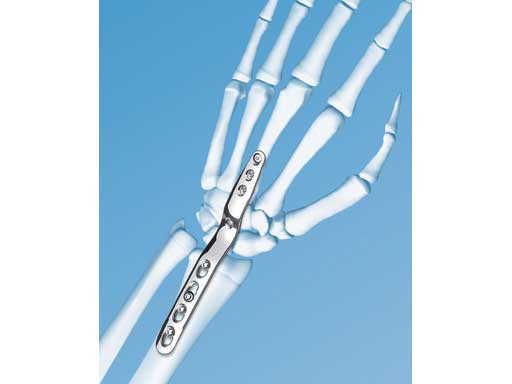
The LCP wrist fusion system offers three different plates: a standard bend plate, a short bend plate, and a straight plate. The correct plate is selected according to the condition of the soft tissues and the boney anatomy of the patient's wrist. The standard bend plate is used for radiometacarpal fixation of average-sized individuals. The short bend plate is used for wrist fixation in small-stature individuals and for fusion following proximal row carpectomy. The straight plate is used for wrist fixation when the standard and short bend plates do not fit the anatomy.
This plate can be contoured according to the specific needs of the anatomy of the patients wrist.
All implants are available in implant quality 316L stainless steel and commercially pure titanium.
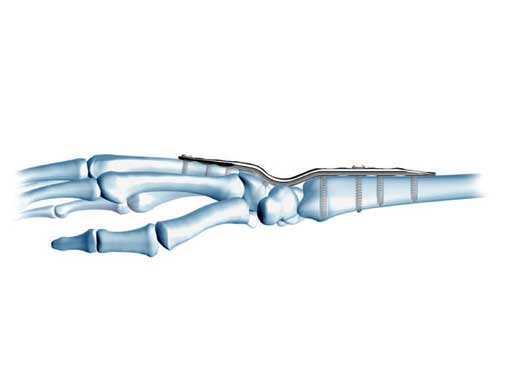
All plates are precontoured, low-profile, and limited-contact. The fusion angle of 10 of dorsiflexion provides optimum hand position. The plate geometry is identical to the original and widely used LC-DCP wrist fusion plate, except for overall length. A combination hole dorsal to the capitate allows lag screw or locking screw fixation of the capitate to the plate. Distally the plates accept 2.7 mm locking and cortex screws and 3.5 mm locking and cortex screws proximally. Locking screws with threaded heads are used in combination holes to create a fixed-angle construct, particularly advantageous to osteopenic bone. Screws are self-tapping for easy insertion. Self-retaining StarDrive recess provides improved torque transmission and increased resistance to stripping.
The evolution of the technology of locked plating systems has extended the useful application of wrist fusion plate fixation to osteopenic and osteoporotic bone. More patients who have sustained the debilitating effects of periarticular inflammation may benefit from improved screw stability in osteopenic bone. Improvements in screw fixation in normal metaphyseal bone allows for a more stable construct with the proximal screw in the metacarpal that is always in the proximal metacarpal metaphysis on the distal arm of the plate. In cases of previous plate fixation on the third metacarpal; residual screw hole voids are present and the amount of bone material available for salvage fixation is compromised. With previously drilled 2.7 mm cortex screws the residual screw hole defect can be reliably filled with a locking screw of the same 2.7 mm system if the plate alignment allows. This provides plate to bone fixation of the new revision plate bone construct that previously would be accomplished by the use of 3.5 mm screws distally and an unacceptably large plate implant on the smaller metacarpal.
The LC-DCP wrist fusion system has provided a unique anatomically contoured implant. The modification of the implant (to allow for locking screw techniques) further extends the usefulness in patients with bone density defects.
Wrist fusion is an effective end-stage treatment for the injured and painful wrist. Multiply operated wrists and distal forearms have significant alterations of bone mass, bone alignment and the norm is not a dense bone structure. LCP wrist fusion plate techniques have extended the technical improvements found in other areas of the skeletal fixation to the special problem of stable fixation in wrist reconstruction. Stable fixation may provide the stability needed for the functional aftercare of the adjacent distal radioulnar joint that additionally sustains posttraumatic arthrofibrosis with the concomitant loss of pronation and supination.
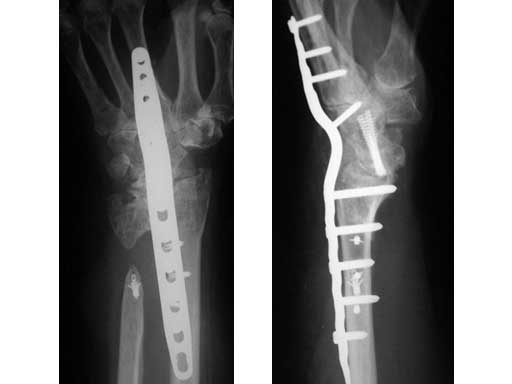
This case example is of a 73-year-old female now 13 years after a distal radius fracture and 2 years after a scapholunate-advanced collapse wrist procedure with Sauve Kapandji arthrodesis for combined posttraumatic instability of the carpus and posttraumatic arthritis of the DRUJ .
The postoperative course for this reconstruction was complicated by a true reflex sympathetic dystrophy (CRPS Type I) responding to stellate ganglion blocks as well as oral pharmaceuticals.
One year before her wrist fusion with LCP wrist fusion plate the patient underwent Achilles tendon allograft for stabilization of an unstable distal forearm articulation. The forearm was stabilized and her pain resolved only to return with radiocarpal crepitation and pain in the terminus of motion in flexion and extension. Her forearm was stable and her hand was warm and supple but she still suffered from rather severe osteopenia and arthrofibrosis of the small finger joints. Her dorsal wrist pain was of sufficient severity, frequency, quality, and location to justify trading her residual wrist motion for pain management. After diagnostic selective wrist joint injections her radiocarpal joint was determined to be a significant source of pain. She underwent successful radiocarpal arthrodesis with local bone graft.
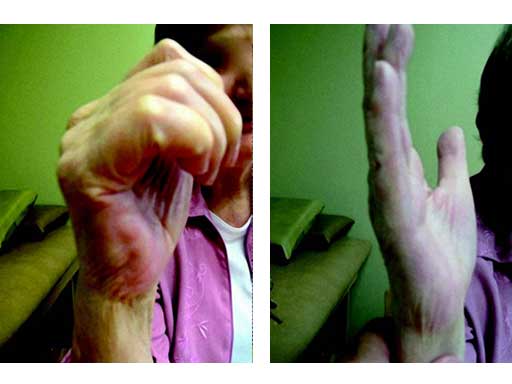
Since wrist fusion her small joint arthrofibrosis of the hand has improved and she is off all pain medications.
Fig 1 Eight weeks after radiocarpal fusion. (left)
Fig 2 One year after distal radius ulnar joint interpostion arthroplasty. (right)
Fig 3ad Functional result 2 years postoperatively.
Fig 3ad Functional result 2 years postoperatively.
Case provided by Thomas Fischer, Indianapolis, USA
Hazards and labeling
Due to varying countries’ legal and regulatory approval requirements, consult the appropriate local product labeling for approved intended use of the products described on this website. All devices on this website are approved by the AO Technical Commission. For logistical reasons, these devices may not be available in all countries worldwide at the date of publication.
Legal restrictions
This work was produced by AO Foundation, Switzerland. All rights reserved by AO Foundation. This publication, including all parts thereof, is legally protected by copyright.
Any use, exploitation or commercialization outside the narrow limits set forth by copyright legislation and the restrictions on use laid out below, without the publisher‘s consent, is illegal and liable to prosecution. This applies in particular to photostat reproduction, copying, scanning or duplication of any kind, translation, preparation of microfilms, electronic data processing, and storage such as making this publication available on Intranet or Internet.
Some of the products, names, instruments, treatments, logos, designs, etc referred to in this publication are also protected by patents, trademarks or by other intellectual property protection laws (eg, “AO” and the AO logo are subject to trademark applications/registrations) even though specific reference to this fact is not always made in the text. Therefore, the appearance of a name, instrument, etc without designation as proprietary is not to be construed as a representation by the publisher that it is in the public domain.
Restrictions on use: The rightful owner of an authorized copy of this work may use it for educational and research purposes only. Single images or illustrations may be copied for research or educational purposes only. The images or illustrations may not be altered in any way and need to carry the following statement of origin “Copyright by AO Foundation, Switzerland”.
Check www.aofoundation.org/disclaimer for more information.
If you have any comments or questions on the articles or the new devices, please do not hesitate to contact us.
“approved by AO Technical Commission” and “approved by AO”
The brands and labels “approved by AO Technical Commission” and “approved by AO”, particularly "AO" and the AO logo, are AO Foundation's intellectual property and subject to trademark applications and registrations, respectively. The use of these brands and labels is regulated by licensing agreements between AO Foundation and the producers of innovation products obliged to use such labels to declare the products as AO Technical Commission or AO Foundation approved solutions. Any unauthorized or inadequate use of these trademarks may be subject to legal action.
AO ITC Innovations Magazine
Find all issues of the AO ITC Innovations Magazine for download here.
Innovation Awards
Recognizing outstanding achievements in development and fostering excellence in surgical innovation.