Orthodontic Bone Anchor
The technique of using a fixed anchorage point for precise orthodontic alignment of posterior teeth has become a standard procedure in orthodontics mainly in Asian countries for more than a decade and is now gaining more popularity in other parts of the world. For the development of the system, feedbacks were collected from more than 100 oral and maxillofacial surgeons and orthodontists working in US , Japan, Canada, Europe and Hong Kong were obtained.
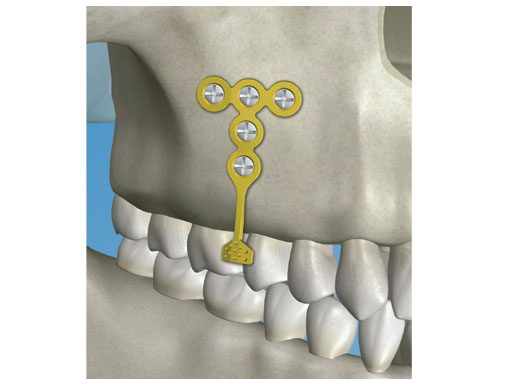
The newly designed orthodontic bone anchor (OBA) system is aimed to be implanted into the jaw bone via an intraoral approach and used as a stable fixation point for the orthodontic procedures. This system eliminates the need for headgear or similar extra-oral anchorage and able to offer more control than the traditional techniques. The system is composed of a variety of screw anchors, plate anchors, and respective instruments for implementation.
The anchor devices of OBA system provide fixed anchorage for improved orthodontic control of tooth movement and enable immediate loading of anchorage force for the teeth to be moved. They can be used in combination with a variety of orthodontic arch wires, elastics and springs.
The screw anchors are available with thread lengths of 6 and 8mm (self-drilling) as well as 10mm and they are all designed for self-tapping. A 1.5 mm non-threaded gingival collar beneath the head was added to protect the soft tissue from compression and prevent burying of the screw heads. All anchor screws have through-holes that accommodate orthodontic wire with a cross section of 0.022 x 0.028 in. The thread diameter of the screws is only1.55mm and this allows for placement into the alveolar bone between the tooth roots.
In cases where a strong fixation is required or more favorable orthodontic vectors are needed, plate anchors are included in the set as well. They can be adapted to the patients bony anatomy and position further away from the tooth roots. The anchor plates can be fixed with self-drilling screws (1.55mm). It is recommended to use at least three screws per plate for optimal fixation. If any screw gets loose upon insertion in the bone, emergency screws (1.85mm) are available in the set for use. The plate anchor neck is malleable to allow for later adjustments if necessary.
All implants are manufactured from commercially pure (CP) titanium and titanium alloy (Ti-6Al-7Nb). The set contains a screw driver handle with hex coupling and the new Matrix Midface screwdriver blade as well as bending pliers and plate cutters.
Not intended for use in:
- Less than 5mm bone thickness or insufficient bone quality
- Deciduous or mixed dentition
- Active or chronic infection
- Abnormal habits of mastication or bruxism
Figure Plate anchor
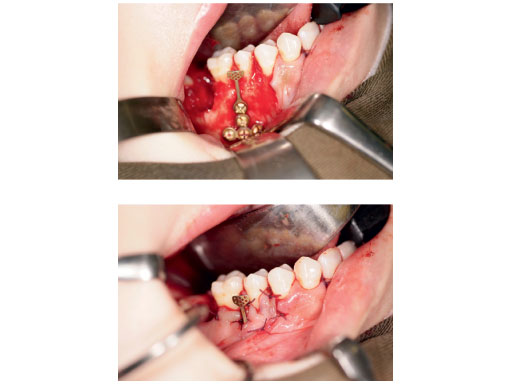
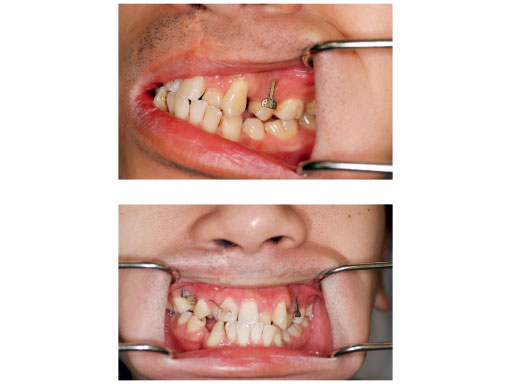
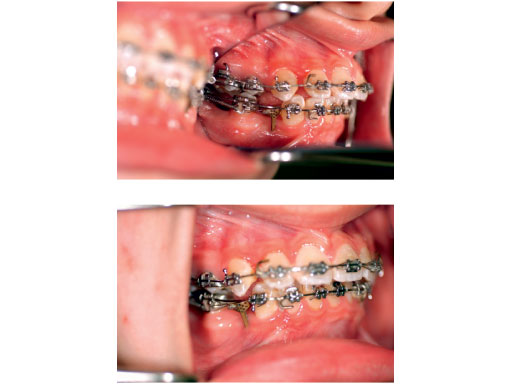
Case images courtesy of Lim K Cheung, Hong Kong, HK
Hazards and labeling
Due to varying countries’ legal and regulatory approval requirements, consult the appropriate local product labeling for approved intended use of the products described on this website. All devices on this website are approved by the AO Technical Commission. For logistical reasons, these devices may not be available in all countries worldwide at the date of publication.
Legal restrictions
This work was produced by AO Foundation, Switzerland. All rights reserved by AO Foundation. This publication, including all parts thereof, is legally protected by copyright.
Any use, exploitation or commercialization outside the narrow limits set forth by copyright legislation and the restrictions on use laid out below, without the publisher‘s consent, is illegal and liable to prosecution. This applies in particular to photostat reproduction, copying, scanning or duplication of any kind, translation, preparation of microfilms, electronic data processing, and storage such as making this publication available on Intranet or Internet.
Some of the products, names, instruments, treatments, logos, designs, etc referred to in this publication are also protected by patents, trademarks or by other intellectual property protection laws (eg, “AO” and the AO logo are subject to trademark applications/registrations) even though specific reference to this fact is not always made in the text. Therefore, the appearance of a name, instrument, etc without designation as proprietary is not to be construed as a representation by the publisher that it is in the public domain.
Restrictions on use: The rightful owner of an authorized copy of this work may use it for educational and research purposes only. Single images or illustrations may be copied for research or educational purposes only. The images or illustrations may not be altered in any way and need to carry the following statement of origin “Copyright by AO Foundation, Switzerland”.
Check www.aofoundation.org/disclaimer for more information.
If you have any comments or questions on the articles or the new devices, please do not hesitate to contact us.
“approved by AO Technical Commission” and “approved by AO”
The brands and labels “approved by AO Technical Commission” and “approved by AO”, particularly "AO" and the AO logo, are AO Foundation's intellectual property and subject to trademark applications and registrations, respectively. The use of these brands and labels is regulated by licensing agreements between AO Foundation and the producers of innovation products obliged to use such labels to declare the products as AO Technical Commission or AO Foundation approved solutions. Any unauthorized or inadequate use of these trademarks may be subject to legal action.
AO ITC Innovations Magazine
Find all issues of the AO ITC Innovations Magazine for download here.
Innovation Awards
Recognizing outstanding achievements in development and fostering excellence in surgical innovation.