Matrix Midface System
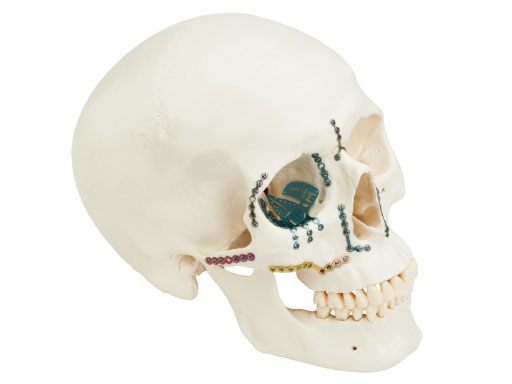
The new matrix midface plate and screw system is intended for use in selective trauma of the midface and craniofacial skeleton; craniofacial surgery; reconstructive procedures; and selective orthognatic surgery of the maxilla and chin. One of the main intentions was to make the system flexible and easy to use.
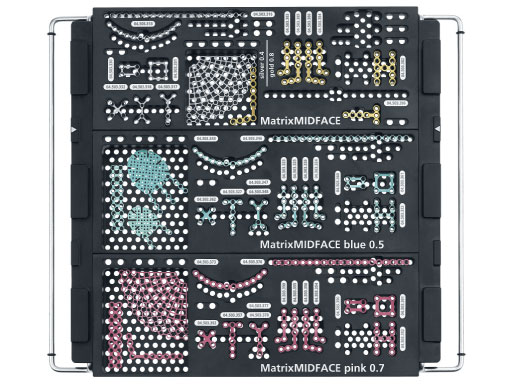
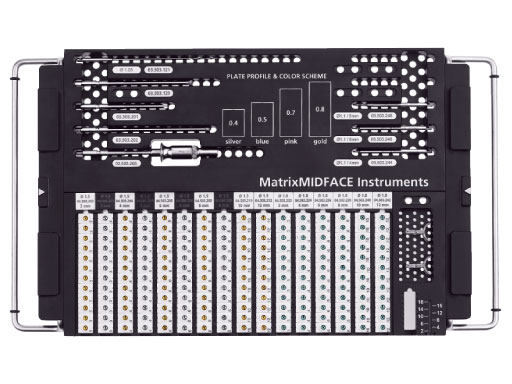
The system offers a full range plate selection for NOE, ZMC, LeFort I, and a variety of other craniofacial indications made out of commercially pure titanium. Four thicknesses are available: 0.4mm (silver), 0.5mm (blue), 0.7 mm (pink), and 0.8mm (gold). The color coding designates the plate strength.
MatrixMIDFACE 1.3 Line Extension, April 2021
In April 2021, the MatrixMIDFACE System was expanded with new 1.3 and 1.5 mm plates and screws. Read more in the "Details" tab below.
Only one screw diameter and one screwdriver blade
To make the system more user friendly and simple it is designed in a way that all screws work with all plates within the entire set. Moreover only one screwdriver blade is necessary as it works for all screws within the system.
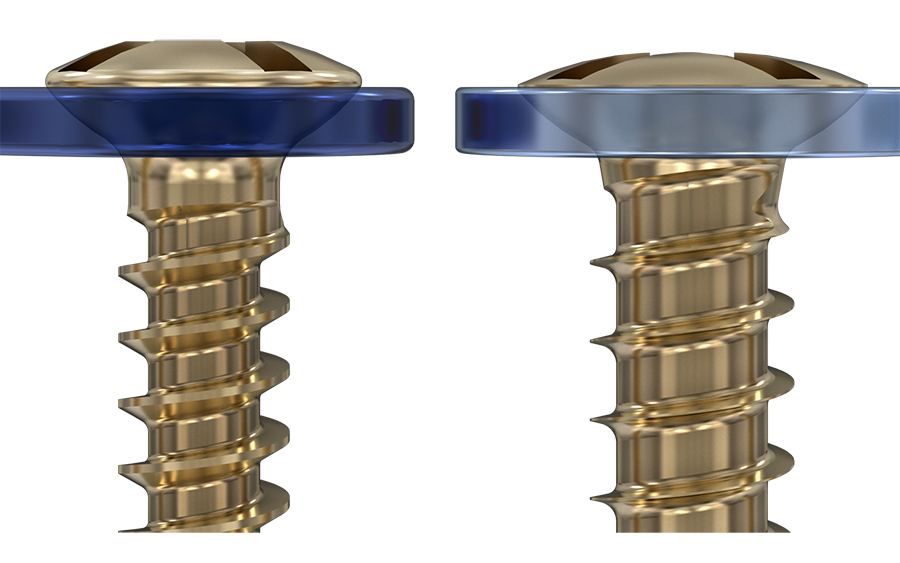
The screws are available in 3mm to 18mm lengths. They also have a color coding to distinguish between self-drilling (silver), self-tapping (bronze), and emergency (blue) designs. In comparison to the existing set, the major advantages include a recess for improved screw driver retention and reduced cam-out, and faster insertion due to the 0.60 mm thread pitch design. All screws in the set are made out of titanium alloy (Ti-6Al-7Nb).
Other improvements are the reduced plate/screw profile and the self retaining screws/blades allowing for easier screw/blade re-engagement and reduced screw cam-out. The edges on the plates are rounded to avoid soft tissue damage. A reduced overall plate and screw profile has been achieved.
The standardized instrumentation makes the matrix midface system more efficient and helps reducing inventory for hospitals without compromising clinical solutions.
As with every AO product, the matrix midface system has been tested at a large number of sites before achieving AO approval. When compared to existing sets, those surveyed reported the new system to be much easier to use and found the consolidation of product that the Matrix system offers to be an important step forward. Additionally, the screw/plate profile and screw insertion/retention were found to be significantly better in comparison to the existing 1.5mm system.
MatrixMIDFACE 1.3 Line Extension
The MatrixMIDFACE System has been expanded with the new plates 1.3 and 1.3 mm screws
In April 2021, the new 1.3 and 1.5 mm plates and screws are aligned with the Matrix concept providing low profile plates and screws in a modular system with a wide range of plate shapes and screw options for fracture fixation. The new 1.3 mm screws (existing Matrix Screws are 1.55 mm in thread diameter) have allowed the distance between the screw holes in the plates 1.3 to be significantly reduced, providing more fixation options in the smaller plates (Fig 1). The 1.3 mm screws are not compatible with the existing Matrix plates 1.5 designed for 1.55 m screws and vice versa. The instrumentation for both Matrix Systems is identical. A smaller 1.0 mm drill bit, required for the 1.3 mm screws, has been included from the COMPACT Midface System. With the inclusion of new plates and screws, the MatrixMIDFACE System supersedes the COMPACT Midface System and offers implants in a range of sizes and shapes suitable for a variety of applications.
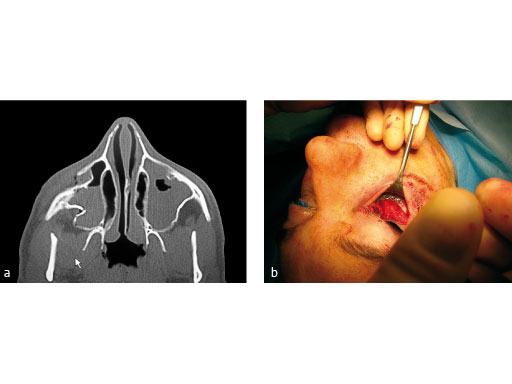
Fig 1ab
Treatment of zygomatic fracture with matrix midface plates.
Case images courtesy of Scott P Bartlett, Philadelphia, US
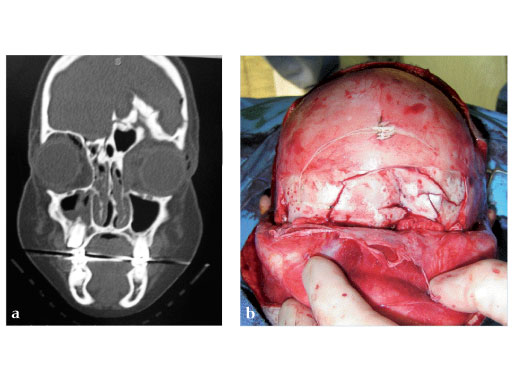
Fig 2ad Highly comminuted panfacial fracture.
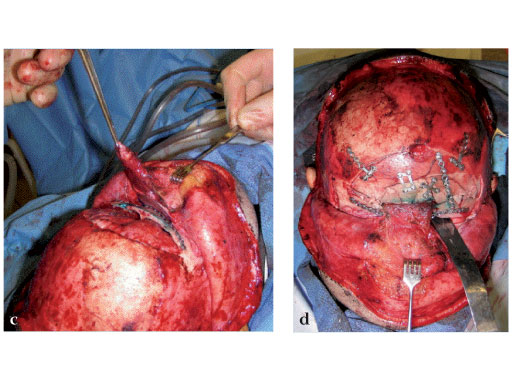
Fig 2ad Highly comminuted panfacial fracture (continued).
Case images courtesy of Scott P Bartlett, Philadelphia, US
Hazards and labeling
Due to varying countries’ legal and regulatory approval requirements, consult the appropriate local product labeling for approved intended use of the products described on this website. All devices on this website are approved by the AO Technical Commission. For logistical reasons, these devices may not be available in all countries worldwide at the date of publication.
Legal restrictions
This work was produced by AO Foundation, Switzerland. All rights reserved by AO Foundation. This publication, including all parts thereof, is legally protected by copyright.
Any use, exploitation or commercialization outside the narrow limits set forth by copyright legislation and the restrictions on use laid out below, without the publisher‘s consent, is illegal and liable to prosecution. This applies in particular to photostat reproduction, copying, scanning or duplication of any kind, translation, preparation of microfilms, electronic data processing, and storage such as making this publication available on Intranet or Internet.
Some of the products, names, instruments, treatments, logos, designs, etc referred to in this publication are also protected by patents, trademarks or by other intellectual property protection laws (eg, “AO” and the AO logo are subject to trademark applications/registrations) even though specific reference to this fact is not always made in the text. Therefore, the appearance of a name, instrument, etc without designation as proprietary is not to be construed as a representation by the publisher that it is in the public domain.
Restrictions on use: The rightful owner of an authorized copy of this work may use it for educational and research purposes only. Single images or illustrations may be copied for research or educational purposes only. The images or illustrations may not be altered in any way and need to carry the following statement of origin “Copyright by AO Foundation, Switzerland”.
Check www.aofoundation.org/disclaimer for more information.
If you have any comments or questions on the articles or the new devices, please do not hesitate to contact us.
“approved by AO Technical Commission” and “approved by AO”
The brands and labels “approved by AO Technical Commission” and “approved by AO”, particularly "AO" and the AO logo, are AO Foundation's intellectual property and subject to trademark applications and registrations, respectively. The use of these brands and labels is regulated by licensing agreements between AO Foundation and the producers of innovation products obliged to use such labels to declare the products as AO Technical Commission or AO Foundation approved solutions. Any unauthorized or inadequate use of these trademarks may be subject to legal action.
AO ITC Innovations Magazine
Find all issues of the AO ITC Innovations Magazine for download here.
Innovation Awards
Recognizing outstanding achievements in development and fostering excellence in surgical innovation.