Expert Hindfoot Arthrodesis Nail (HAN)
The Expert hindfoot arthrodesis nail (HAN) is indicated to facilitate tibiotalocalcaneal arthrodesis to treat severe foot/ankle deformity, arthritis, instability, and skeletal defects; these include, but are not limited to neuro-osteoarthropathy (Charcot foot), avascular necrosis of the talus, failed joint replacement or failed ankle fusion, distal tibial fracture nonunions, and osteoarthritis, rheumatoid arthritis and pseudoarthrosis, and tumor resection and reconstruction.
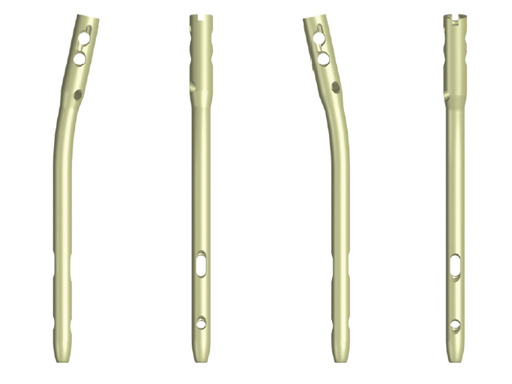
The cannulated hindfoot arthrodesis nail has a lateral bend of 12 which allows an entry point in the center of the lateral column of the calcaneus. The nail enables proper hindfoot alignment and restores 35 hindfoot valgus positioning.
The nail design, a rotating insertion handle and the aiming arm enable targeted medial-to-lateral or lateral-to-medial proximal locking. Distal locking options are with spiral blade in the calcaneus for more stability in osteopenic bone, with a 6.0?mm Stardrive locking head screw from the calcaneus into the cuboid, or with a 5.0?mm Stardrive locking head screw from the talus into the navicular or further distal in the medial column. An end cap for screws and for spiral blades is available.
As part of the Expert nail family, the HAN uses most of the instrumentation of the humeral, femoral and tibial nails, except for an extended length 5.0?mm calibrated drill for extra long 6.0?mm locking head screws.
The nails are available in 10, 12 and 13?mm diameters, in 150, 180, and 240?mm lengths, right and left designs.
Study
The objectives of this retrospective study were to document the current clinical experience gained from the use of the HAN in the treatment of patients with various ankle and foot pathologies.
In addition, information was collected relating to surgical details, functional and quality of life outcomes after HAN procedures (SF-36, AAOS-FAOQ and numeric rating scale of pain). Seven participating clinics (four from Europe and three from the USA) recruited 38 patients who underwent ankle arthrodesis using the HAN. A large socioeconomic benefit appears to stem from hindfoot fusion with the HAN in properly selected patients because a high proportion of patients who were on sick leave prior to surgery were able to return to work. Early findings appear to indicate that HAN offers a safe and reliable salvage option for patients undergoing tibiotalocalcaneal arthrodesis. More detailed information on the results will be released in a full manuscript to be submitted for peer review in late 2011.
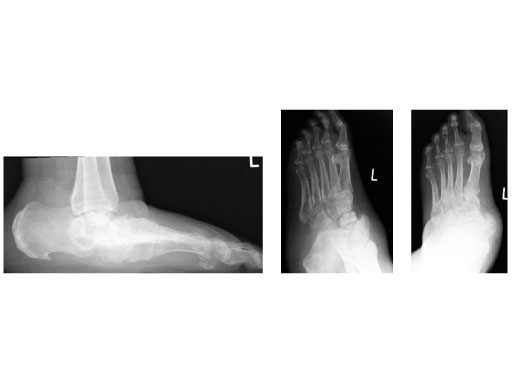
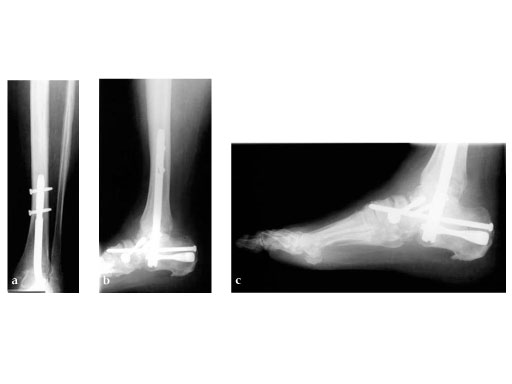
42-year-old, female with severe insulin-dependent diabetes mellitus (IDDM); Charcot foot, wheel chair since 1 year.
Case provided by Hermann Bail, Berlin, DE
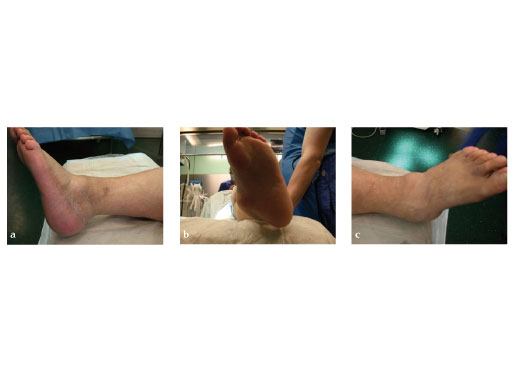
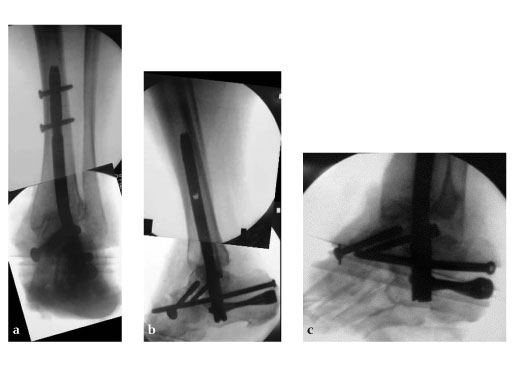
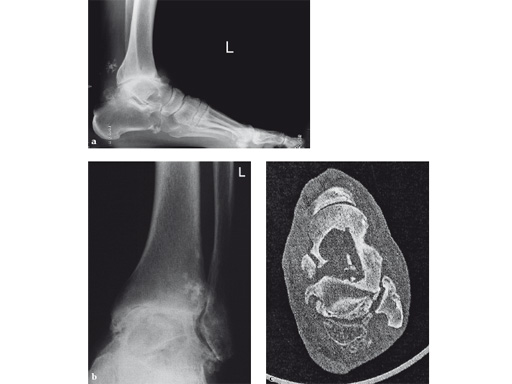
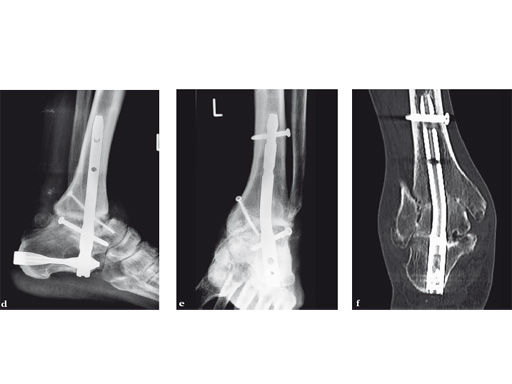
A 64-year-old man presented with severe ankle and subtalar arthritis accompanied by large cysts in the talus and calcaneus. Eighteen months after hindfoot fusion with the HAN he walked without pain.
Case provided by Stefan Rammelt, Dresden, Germany
Hazards and labeling
Due to varying countries’ legal and regulatory approval requirements, consult the appropriate local product labeling for approved intended use of the products described on this website. All devices on this website are approved by the AO Technical Commission. For logistical reasons, these devices may not be available in all countries worldwide at the date of publication.
Legal restrictions
This work was produced by AO Foundation, Switzerland. All rights reserved by AO Foundation. This publication, including all parts thereof, is legally protected by copyright.
Any use, exploitation or commercialization outside the narrow limits set forth by copyright legislation and the restrictions on use laid out below, without the publisher‘s consent, is illegal and liable to prosecution. This applies in particular to photostat reproduction, copying, scanning or duplication of any kind, translation, preparation of microfilms, electronic data processing, and storage such as making this publication available on Intranet or Internet.
Some of the products, names, instruments, treatments, logos, designs, etc referred to in this publication are also protected by patents, trademarks or by other intellectual property protection laws (eg, “AO” and the AO logo are subject to trademark applications/registrations) even though specific reference to this fact is not always made in the text. Therefore, the appearance of a name, instrument, etc without designation as proprietary is not to be construed as a representation by the publisher that it is in the public domain.
Restrictions on use: The rightful owner of an authorized copy of this work may use it for educational and research purposes only. Single images or illustrations may be copied for research or educational purposes only. The images or illustrations may not be altered in any way and need to carry the following statement of origin “Copyright by AO Foundation, Switzerland”.
Check www.aofoundation.org/disclaimer for more information.
If you have any comments or questions on the articles or the new devices, please do not hesitate to contact us.
“approved by AO Technical Commission” and “approved by AO”
The brands and labels “approved by AO Technical Commission” and “approved by AO”, particularly "AO" and the AO logo, are AO Foundation's intellectual property and subject to trademark applications and registrations, respectively. The use of these brands and labels is regulated by licensing agreements between AO Foundation and the producers of innovation products obliged to use such labels to declare the products as AO Technical Commission or AO Foundation approved solutions. Any unauthorized or inadequate use of these trademarks may be subject to legal action.
AO ITC Innovations Magazine
Find all issues of the AO ITC Innovations Magazine for download here.
Innovation Awards
Recognizing outstanding achievements in development and fostering excellence in surgical innovation.