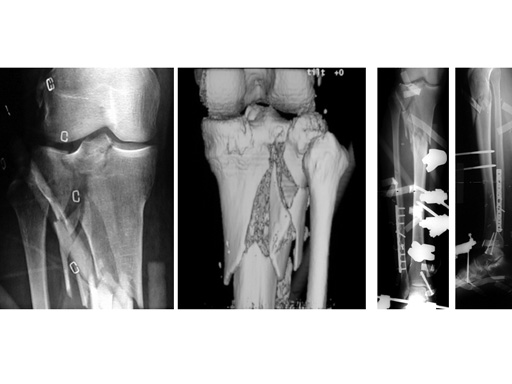
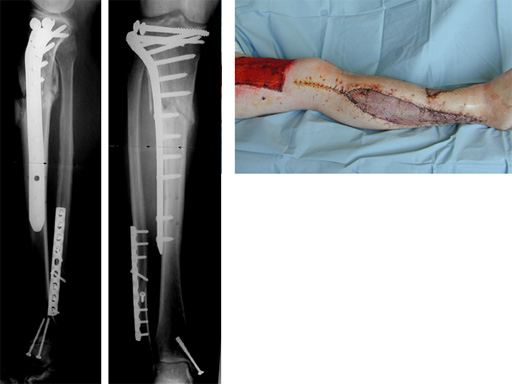
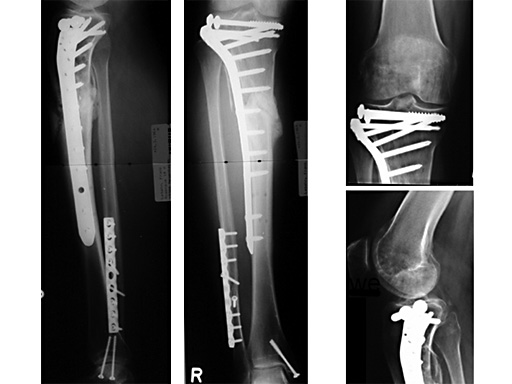
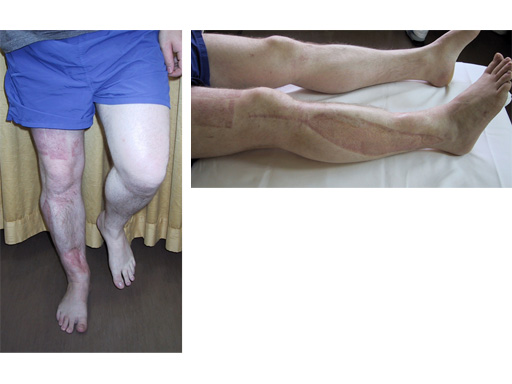
LISS Proximal Lateral Tibia: Study Results
The LIS system for the proximal tibia is an anatomically pre-contoured implant for the treatment of fractures of the proximal tibia. In the course of a prospective clinical study, 135 patients with 138 fractures were treated with the new system at 13 European trauma centers from June 1998 to March 2000 and the cases were documented. The follow-up period was at least 12 months and ended in June 2001. Principle clinical investigator is C. Krettek, Hanover. AOCID monitored the study.
The inclusion criteria took in fractures of all degrees of severity of the proximal tibia and the tibial shaft. The fractures were classified as 110 fractures of the proximal tibia (AO 41) and 28 tibial shaft fractures (AO 42) according to the AO fracture classification system; type C fractures accounted for a total of 66% (n=91). A follow-up rate of 94% was achieved with reference to the number of fractures treated. The results of the study were evaluated immediately in close collaboration with the Principal Clinical Investigator. It is expected that the evaluation will be completed by the end of 2001; the results will be summarized and published in a final report.
Hazards and labeling
Due to varying countries’ legal and regulatory approval requirements, consult the appropriate local product labeling for approved intended use of the products described on this website. All devices on this website are approved by the AO Technical Commission. For logistical reasons, these devices may not be available in all countries worldwide at the date of publication.
Legal restrictions
This work was produced by AO Foundation, Switzerland. All rights reserved by AO Foundation. This publication, including all parts thereof, is legally protected by copyright.
Any use, exploitation or commercialization outside the narrow limits set forth by copyright legislation and the restrictions on use laid out below, without the publisher‘s consent, is illegal and liable to prosecution. This applies in particular to photostat reproduction, copying, scanning or duplication of any kind, translation, preparation of microfilms, electronic data processing, and storage such as making this publication available on Intranet or Internet.
Some of the products, names, instruments, treatments, logos, designs, etc referred to in this publication are also protected by patents, trademarks or by other intellectual property protection laws (eg, “AO” and the AO logo are subject to trademark applications/registrations) even though specific reference to this fact is not always made in the text. Therefore, the appearance of a name, instrument, etc without designation as proprietary is not to be construed as a representation by the publisher that it is in the public domain.
Restrictions on use: The rightful owner of an authorized copy of this work may use it for educational and research purposes only. Single images or illustrations may be copied for research or educational purposes only. The images or illustrations may not be altered in any way and need to carry the following statement of origin “Copyright by AO Foundation, Switzerland”.
Check www.aofoundation.org/disclaimer for more information.
If you have any comments or questions on the articles or the new devices, please do not hesitate to contact us.
“approved by AO Technical Commission” and “approved by AO”
The brands and labels “approved by AO Technical Commission” and “approved by AO”, particularly "AO" and the AO logo, are AO Foundation's intellectual property and subject to trademark applications and registrations, respectively. The use of these brands and labels is regulated by licensing agreements between AO Foundation and the producers of innovation products obliged to use such labels to declare the products as AO Technical Commission or AO Foundation approved solutions. Any unauthorized or inadequate use of these trademarks may be subject to legal action.
AO ITC Innovations Magazine
Find all issues of the AO ITC Innovations Magazine for download here.
Innovation Awards
Recognizing outstanding achievements in development and fostering excellence in surgical innovation.