LCP Distal Femur
The LCP Distal Femur is a pre-shaped, low profile plate combining the successful LISS DF with the Combination Hole concept. The LCP Distal Femur is indicated for distal shaft fractures, supracondylar fractures, intraarticular fractures, and periprosthetic fractures. The plates are available in a left and right version, both with 513 holes. The implants and instruments of the LCP Distal Femur are fully compatible with the 4.5/5.0 mm LCP Systems.
LCP DF: 15-19 Holes
The LCP distal femur (DF) is indicated for distal shaft, supracondylar, intraarticular, and extraarticular, as well as periprosthetic fractures. Until recently, the plates were available in lengths up to 13 holes, with the longest plates 300 mm. Longer plates may be useful for appropriate bridging of comminuted fractures or for spanning a hip prosthesis to avoid stress risers in the proximal femur. For the treatment of these fracture types, additional plate lengths of 15, 17, and 19 holes with a maximum length of 436 mm are now available in left and right versions. They have the same design as the existing 513-hole plates and can be used with the same aiming arm, instruments, and screws.
LISS Distal Femur: Study Results
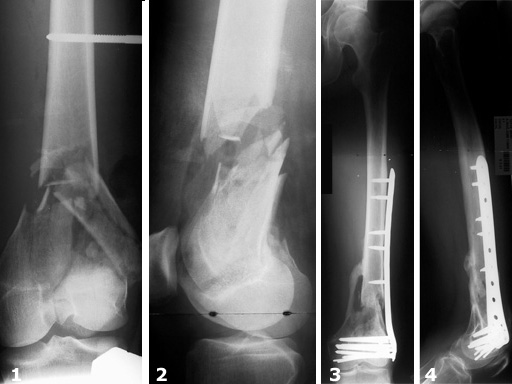
The LIS System Distal Femur (DF) is a new, fixed-angle implant system for the treatment of distal femoral fractures according to the principles of Minimally Invasive Surgery.
From December 1996 to November 1998 116 fractures (112 patients) were treated with the new stabilization system as part of a prospective multicenter study. The follow-up period was 13.7 months on average. Principle clinical investigator was N. Haas, Berlin. The criteria for inclusion were distal femoral shaft fractures and supracondylar and intraarticular femoral fractures of all degrees of severity. In this study, thirty-one distal femoral shaft fractures and eighty-five supracondylar and/or intraarticular femoral fractures were treated. There was a total of 45% (n=52) complex intraarticular fractures. With reference to the fractures treated, the follow-up rate was 93%. In 90% of the cases treated and followed up, fracture consolidation during the observation period could be confirmed.
Secondary cancellous bone grafting was only necessary in six cases. There were four cases of infection requiring surgical and antibiotic treatment. Implant loosening was observed in four cases, some of which could be attributed to technical errors during the operation. In contrast, secondary depressions during the course of healing were not observed at all.
The results of the study show that the new internal fixator is an excellent, safe procedure for the treatment of almost all fracture types, provided that thorough preoperative planning is performed and the surgeon has a sound knowledge of the operative technique. There is generally no need for primary cancellous bone grafting.
A Guiding Block is available for both left and right versions.
Female, 27 years
Hazards and labeling
Due to varying countries’ legal and regulatory approval requirements, consult the appropriate local product labeling for approved intended use of the products described on this website. All devices on this website are approved by the AO Technical Commission. For logistical reasons, these devices may not be available in all countries worldwide at the date of publication.
Legal restrictions
This work was produced by AO Foundation, Switzerland. All rights reserved by AO Foundation. This publication, including all parts thereof, is legally protected by copyright.
Any use, exploitation or commercialization outside the narrow limits set forth by copyright legislation and the restrictions on use laid out below, without the publisher‘s consent, is illegal and liable to prosecution. This applies in particular to photostat reproduction, copying, scanning or duplication of any kind, translation, preparation of microfilms, electronic data processing, and storage such as making this publication available on Intranet or Internet.
Some of the products, names, instruments, treatments, logos, designs, etc referred to in this publication are also protected by patents, trademarks or by other intellectual property protection laws (eg, “AO” and the AO logo are subject to trademark applications/registrations) even though specific reference to this fact is not always made in the text. Therefore, the appearance of a name, instrument, etc without designation as proprietary is not to be construed as a representation by the publisher that it is in the public domain.
Restrictions on use: The rightful owner of an authorized copy of this work may use it for educational and research purposes only. Single images or illustrations may be copied for research or educational purposes only. The images or illustrations may not be altered in any way and need to carry the following statement of origin “Copyright by AO Foundation, Switzerland”.
Check www.aofoundation.org/disclaimer for more information.
If you have any comments or questions on the articles or the new devices, please do not hesitate to contact us.
“approved by AO Technical Commission” and “approved by AO”
The brands and labels “approved by AO Technical Commission” and “approved by AO”, particularly "AO" and the AO logo, are AO Foundation's intellectual property and subject to trademark applications and registrations, respectively. The use of these brands and labels is regulated by licensing agreements between AO Foundation and the producers of innovation products obliged to use such labels to declare the products as AO Technical Commission or AO Foundation approved solutions. Any unauthorized or inadequate use of these trademarks may be subject to legal action.
AO ITC Innovations Magazine
Find all issues of the AO ITC Innovations Magazine for download here.
Innovation Awards
Recognizing outstanding achievements in development and fostering excellence in surgical innovation.