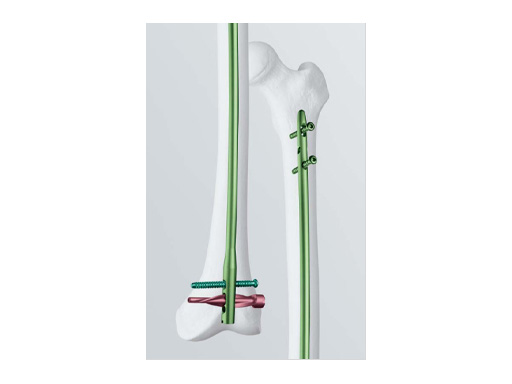
Distal Femoral Nail with Spiral Blade
In the past years, retrograde femoral nailing has proven to be safe and reliable. The Distal Femoral Nail is becoming increasingly popular and is especially suitable for obese (or pregnant) patients and in osteoporotic metaphyseal bone.
Indications include extra-articular metaphyseal distal femoral fractures (32-A1 to C3) and supracondylar fractures and simple articular fractures (33-A1 to A3 and 33-C1 to C3.1).
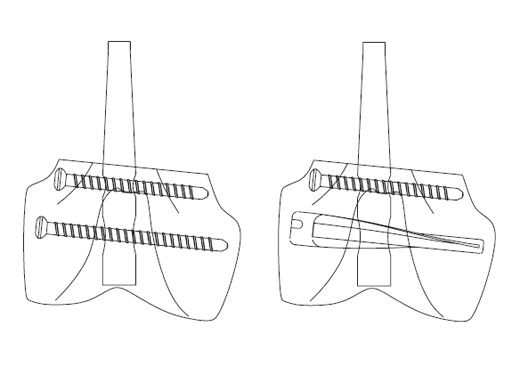
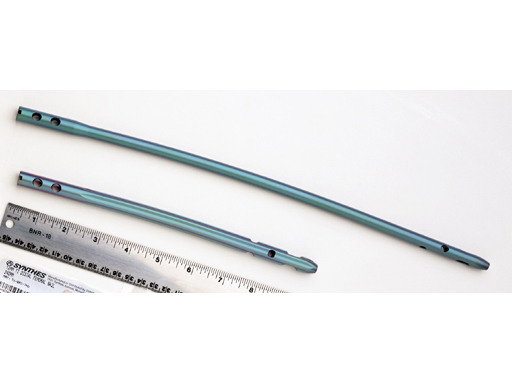
The Spiral Blade enables significantly more stable distal interlocking than with conventional locking bolts due to the large load-bearing surface. It reduces cancellous bone compaction and lowers the risk of nail protrusion into the knee joint. The nail diameters are 9.0 and 10.0 mm for solid nails and 12.0 mm for cannulated nails.
Lengths range from 160 to 420mm.
A Scientific Study from the AO Research Institute
Objective: Intramedullary nail locking bolts often fail to gain purchase or cut out in osteoporotic bone. The biomechanical stability of a bladelike device that lowers intraosseous stress levels by distributing the load over a greater volume of bone was compared with conventional locking bolts in osteoporotic bone.
Methods: Standardized simulated comminuted supracondylar femoral fractures (segmental defect) in fresh-frozen paired osteoporotic (bone mineral density <200 milligrams per cubic centimeter) human cadaveric femurs were stabilized with a retrograde unreamed distal femoral nail and distally interlocked with conventional locking bolts or a bladelike device. The distal portions of the fixator-bone constructs were tested under axial load, and the stiffness and strength were compared (pairwise).
Results: Interlocking with a bladelike device was 41 percent stiffer (p = 0.01) and 20 percent stronger (p = 0.02) than that with conventional locking bolts. All posttesting radiographs showed compaction of the cancellous bone distal to the interlocking devices. Even after nail displacements of twelve millimeters, only a few locking bolts were plastically deformed and no bladelike device showed gross plastic deformation.
Conclusion: This study showed the biomechanical benefits of increasing the bone-implant interface surface for improving the acute stiffness and strength of fracture fixation in osteoporotic cancellous bone. The fixator-bone construct withstood higher forces before failure in these fragile bones.
Additional Nail Lengths and 6.0 mm Ti Locking Screws
The Titanium Distal Femoral Nail (DFN) is now available in additional sizes of 180 mm, 220 mm, and 260 mm, in all diameters, to better accommodate patients anatomy.
The existing 6.0 mm Ti Locking Screw line has been extended with sizes 30 mm, 35 mm, and 40 mm for smaller patients.
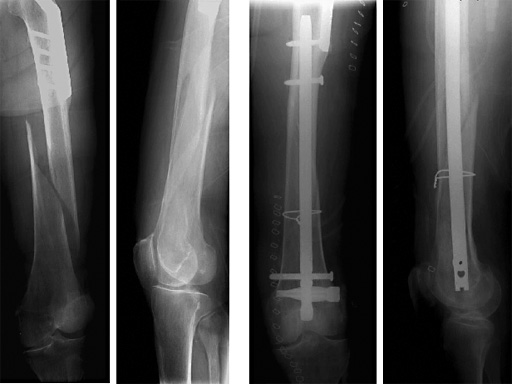
Female, 88 years
Hazards and labeling
Due to varying countries’ legal and regulatory approval requirements, consult the appropriate local product labeling for approved intended use of the products described on this website. All devices on this website are approved by the AO Technical Commission. For logistical reasons, these devices may not be available in all countries worldwide at the date of publication.
Legal restrictions
This work was produced by AO Foundation, Switzerland. All rights reserved by AO Foundation. This publication, including all parts thereof, is legally protected by copyright.
Any use, exploitation or commercialization outside the narrow limits set forth by copyright legislation and the restrictions on use laid out below, without the publisher‘s consent, is illegal and liable to prosecution. This applies in particular to photostat reproduction, copying, scanning or duplication of any kind, translation, preparation of microfilms, electronic data processing, and storage such as making this publication available on Intranet or Internet.
Some of the products, names, instruments, treatments, logos, designs, etc referred to in this publication are also protected by patents, trademarks or by other intellectual property protection laws (eg, “AO” and the AO logo are subject to trademark applications/registrations) even though specific reference to this fact is not always made in the text. Therefore, the appearance of a name, instrument, etc without designation as proprietary is not to be construed as a representation by the publisher that it is in the public domain.
Restrictions on use: The rightful owner of an authorized copy of this work may use it for educational and research purposes only. Single images or illustrations may be copied for research or educational purposes only. The images or illustrations may not be altered in any way and need to carry the following statement of origin “Copyright by AO Foundation, Switzerland”.
Check www.aofoundation.org/disclaimer for more information.
If you have any comments or questions on the articles or the new devices, please do not hesitate to contact us.
“approved by AO Technical Commission” and “approved by AO”
The brands and labels “approved by AO Technical Commission” and “approved by AO”, particularly "AO" and the AO logo, are AO Foundation's intellectual property and subject to trademark applications and registrations, respectively. The use of these brands and labels is regulated by licensing agreements between AO Foundation and the producers of innovation products obliged to use such labels to declare the products as AO Technical Commission or AO Foundation approved solutions. Any unauthorized or inadequate use of these trademarks may be subject to legal action.
AO ITC Innovations Magazine
Find all issues of the AO ITC Innovations Magazine for download here.
Innovation Awards
Recognizing outstanding achievements in development and fostering excellence in surgical innovation.