Bone substitution materials: chronOs, CEROS
This article provides more detailed information about a synthetic, resorbable, and osteoconductive bone substitute material.
Introduction
Although bone has the capacity to repair and remodel in the healing of fractures, lack of sufficient quantities of bone during trauma or major reconstructive procedures is particularly problematic for orthopedic surgeons.
Bone substitutes play an important role in supporting the growth of new bone across those critical osseous defects. Autograft procedures still represent more than half of the bone grafting procedures and are considered to be the most successful bone graft material (gold standard) because they have osteoconductive and osteoinductive properties. However, bone graft harvesting from the iliac crest requires a second incision and adds significant morbidity. Apart from autografts, allografts, and xenografts, synthetic materials are becoming increasingly popular. These materials are favored because of the absence of disease transmission due to their synthetic origin.
Ceramics
Ceramics are highly crystalline structures formed by heating non-metallic mineral salts to high temperatures (>1000C) in a process known as sintering. Chemical content and purity during manufacturing have to comply with the ASTM standards. The use of ceramics, especially calcium phosphates (CaP), is motivated by the fact that the primary inorganic component of bone is hydroxyapatite (HA, Ca5(PO4)3OH), a subset of the CaP group. However, being the most stable CaP in terms of solubility, HA can be regarded as non degradable over years [1]. On the other hand, the CaP ratio of beta tricalcium phosphate ( - TCP, Ca3(PO4)2) makes it a degradable material by osteoclastic activity.
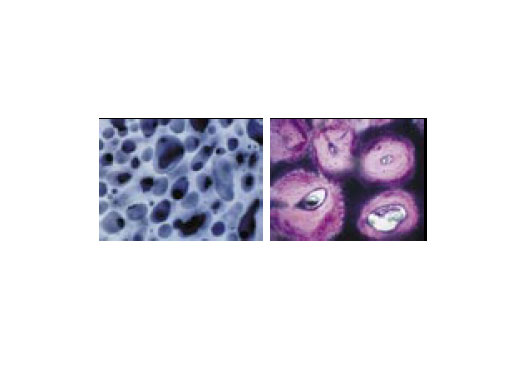
The AO pointed the way to the future in the field of bone substitution materials as early as in the mid-eighties and since then scientific investigations have continuously permitted improvement in their clinical use. The first bone substitutes approved by the AOTK in 1987 were CEROS 80 ( -TCP) and CEROS 82 (HA), both ceramic-based materials. In later years, the interconnecting structure of the implant materials was improved to obtain optimal osteoconductive properties. Thus chronOS was born and quickly became another implant material. With its open-porous and interconnecting structure, chronOS allows blood vessels and the host tissue to gradually grow into the pores (Fig.1). This way, the requirements of an osteoconductive bone substitute are met and the material can be gradually resorbed via physiological metabolic routes.
Clinical applications
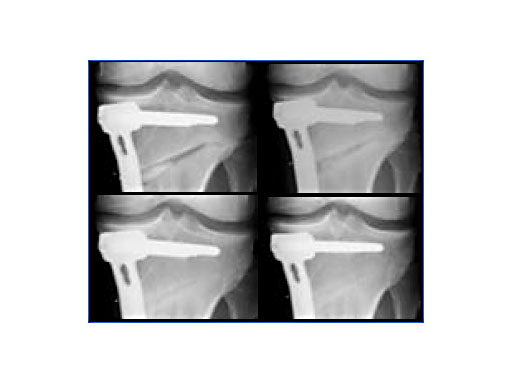
New implant developments are all based on comprehensive in-vitro and in-vivo investigations [2]. In open-wedge high tibial osteotomies, chronOS wedges are well suited for bridging, filling, and correcting bone defects when associated with a stable fixation system [3]. It has been observed that after twelve months various amounts of chronOS were completely resorbed and replaced by new bone (Fig.2).
In spinal surgery, chronOS was used in dorsal spondylodesis, where a large amount of bone graft is needed [4]. In interbody fusion (IBF), the AO is currently testing chronOS pre-filled IBF cages. First clinical results in cervical anterior fusion using the hybrid radiolucent implant CERVIOSchronOS already show excellent fusion results after three months [5]. In the lumbar spine, clinical investigations are still ongoing. The new hybrid IBF implants not only allow avoidance of an additional bone graft harvesting procedure causing patient morbidity, but also significantly reduce both surgery time and blood loss.
In the near future, a solution for the increasing clinical need for injectable and also resorbable materials such as bone void fillers will be offeredchronOS Inject, which is a composite material of brushite, a resorbable CaP phase also found in the body, and -TCP granules. Based on extensive in-vivo studies [6], this new bone substitute is currently being investigated in a clinical study performed by AO CID. More information will be provided soon.
Conclusion
In summary, new advances in material science are permitting mimicry of bone tissue and helping to improve the surgical outcome for the patient. Being synthetic, resorbable, and osteoconductive, chronOS meets the requirements of a reliable bone substitute.
Extensive investigations are currently underway to obtain osteoinductive properties. On the one hand, the temperature at which porous ceramics are sintered can affect biological response by altering the chemical and topographical features of the material surface. Crystal size and form may influence the cell and tissue response by enhancing the adsorption of proteins to the surface and the ability of osteogenic cells to attach, differentiate, and proliferate.
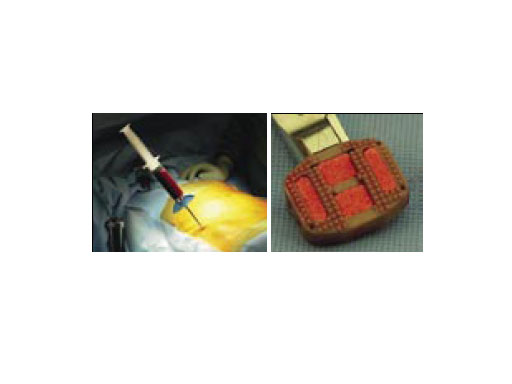
On the other hand, it may be possible with simple intraoperative manipulations to impregnate chronOS with autologous wound healing promoting growth factors released from activated blood platelets and/or osteoprogenitor cells from bone marrow (Fig.3). Thus it may be possible to obtain an osteoinductive bone substitute by autologous means. Promising projects are ongoing at the AO Research Institute in Davos to quantify and understand the osteogenic potential of various autologous substances with a view to clinical application.
Fig.3: Bone marrow puncture from the iliac crest and spinal IBF implant impregnation before implantation, [Dr. P. Pavlov, SMK Nijmegen/NL].
References
1. Bohner M (2000) Calcium orthophosphates in medicine. Injury; 31 (suppl 4).
2. Steffen T et al (2001) Porous tricalcium phosphate and transforming growth factor used for anterior spine surgery. Eur Spine J; 10: 132140.
3. van Hemert W et al Granule or rigid wedge tricalcium phosphate in open wedge high tibial osteotomy. Submitted for publication.
4. Muschik M et al (2001) b-tricalcium phosphate as a bone substitute for dorsal spinal fusion in adolescent idiopathic scoliosis: preliminary results of a prospective clinical study. Eur Spine J; 10: 178184.
5. Pavlov P (2003) Anterior decompression for cervical spondylotic myelopathy. Eur Spine J; 12 (suppl 2): 188194.
6. Bohner M et al (2003) Compositional changes of a dicalcium phosphate dehydrate cement after implantation in sheep. Biomaterials; 24: 34633474.
Biomaterials in Spine Surgery
Hazards and labeling
Due to varying countries’ legal and regulatory approval requirements, consult the appropriate local product labeling for approved intended use of the products described on this website. All devices on this website are approved by the AO Technical Commission. For logistical reasons, these devices may not be available in all countries worldwide at the date of publication.
Legal restrictions
This work was produced by AO Foundation, Switzerland. All rights reserved by AO Foundation. This publication, including all parts thereof, is legally protected by copyright.
Any use, exploitation or commercialization outside the narrow limits set forth by copyright legislation and the restrictions on use laid out below, without the publisher‘s consent, is illegal and liable to prosecution. This applies in particular to photostat reproduction, copying, scanning or duplication of any kind, translation, preparation of microfilms, electronic data processing, and storage such as making this publication available on Intranet or Internet.
Some of the products, names, instruments, treatments, logos, designs, etc referred to in this publication are also protected by patents, trademarks or by other intellectual property protection laws (eg, “AO” and the AO logo are subject to trademark applications/registrations) even though specific reference to this fact is not always made in the text. Therefore, the appearance of a name, instrument, etc without designation as proprietary is not to be construed as a representation by the publisher that it is in the public domain.
Restrictions on use: The rightful owner of an authorized copy of this work may use it for educational and research purposes only. Single images or illustrations may be copied for research or educational purposes only. The images or illustrations may not be altered in any way and need to carry the following statement of origin “Copyright by AO Foundation, Switzerland”.
Check www.aofoundation.org/disclaimer for more information.
If you have any comments or questions on the articles or the new devices, please do not hesitate to contact us.
“approved by AO Technical Commission” and “approved by AO”
The brands and labels “approved by AO Technical Commission” and “approved by AO”, particularly "AO" and the AO logo, are AO Foundation's intellectual property and subject to trademark applications and registrations, respectively. The use of these brands and labels is regulated by licensing agreements between AO Foundation and the producers of innovation products obliged to use such labels to declare the products as AO Technical Commission or AO Foundation approved solutions. Any unauthorized or inadequate use of these trademarks may be subject to legal action.
AO ITC Innovations Magazine
Find all issues of the AO ITC Innovations Magazine for download here.
Innovation Awards
Recognizing outstanding achievements in development and fostering excellence in surgical innovation.