SynCage-LR
SynCage-LR is intended for use in the lumbar spine to facilitate fusion of two adjacent vertebrae. Indications are lumbar and lumbosacral pathologies which may require anterior segmental arthrodesis, including degenerative disc disease and instability, revision surgery for failed decompression syndrome or pseudarthrosis, and reduced spondylolisthesis.
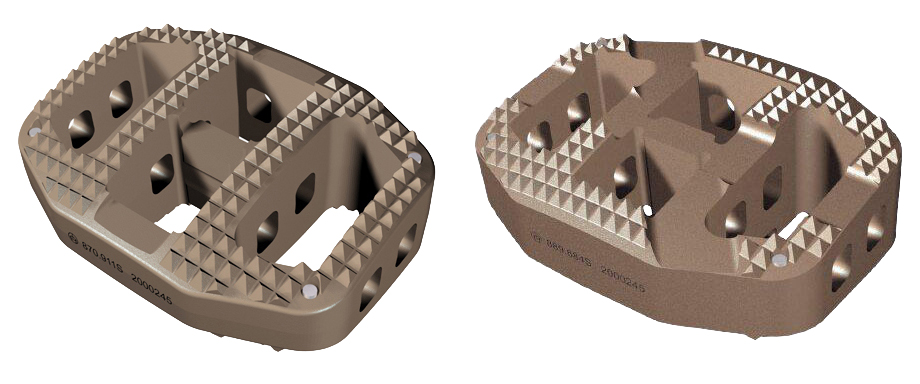
The design of the SynCage-LR features convex superior and inferior surfaces which mimic the natural endplate curvature. Its wedge-shaped body design helps to restore the natural lordotic curvature of the spine and to restore the disc height. The surfaces feature large holes for bony in-growth or chronOS prefilling mass. The pattern of the holes and the large contact surface of the implant with the anatomical contour of the endplate interface serve to reduce the risk of subsidence and to transfer load to the strong peripheral bone of the vertebral body. The SynCage-LR is radiolucent and made out of PEEK Optima. It is available in five heights and two footprints. For the L5S1 segment a special wedge angle, the SynCage-LSR, is available. An additional posterior fixation increases the biomechanical situation significantly.
SynCage-LR 45/90 is a radiolucent SynCage to facilitate fusion of two adjacent vertebrae in the lumbar spine. It is a line extension of the existing SynCage-LR family for anterolateral and lateral approaches. The intended use is not a stand alone application. An additional posterior fixation to improve the biomechanical stability.
The SynCage-LR features convex superior and inferior surfaces which mimic the natural endplate curvature. Its wedge-shaped body design helps to restore the natural lordotic curvature of the spine and to restore the disc height. Dedicated rails for distractor blades allow an anterolateral or true lateral insertion.
The SynCage-LR 45/90 made of PEEK Optima LT3 is available in two footprints with sizes from 1219 mm. The existing instruments of the SynCage-LR family can be used. New Trail Implants permit the precise selection of the implant size and can serve to open the disc space if an anterolateral or true lateral approach is chosen.
“approved by AO Technical Commission” and “approved by AO”
The brands and labels “approved by AO Technical Commission” and “approved by AO”, particularly "AO" and the AO logo, are AO Foundation's intellectual property and subject to trademark applications and registrations, respectively. The use of these brands and labels is regulated by licensing agreements between AO Foundation and the producers of innovation products obliged to use such labels to declare the products as AO Technical Commission or AO Foundation approved solutions. Any unauthorized or inadequate use of these trademarks may be subject to legal action.
AO ITC Innovations Magazine
Find all issues of the AO ITC Innovations Magazine for download here.
Innovation Awards
Recognizing outstanding achievements in development and fostering excellence in surgical innovation.