PLIVIOS
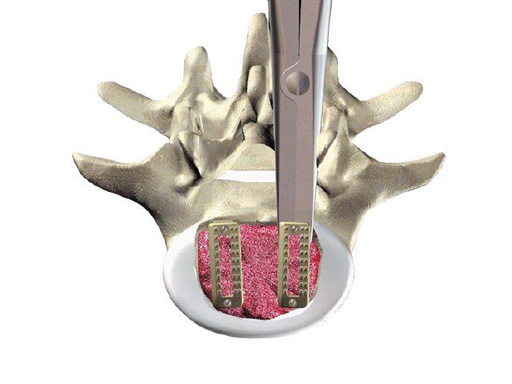
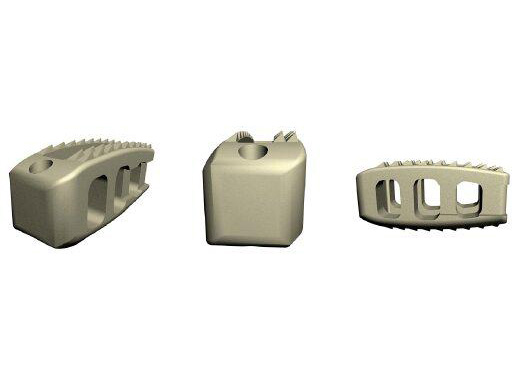
For the surgical treatment of degenerated lumbar discs, cages can be used in order to restore disc height and the lordotic curve. A mechanically stable and durable device such as PLIVIOS or Contact Fusion Cage can be used. The new PLIVIOS implants are based on the clinically established PLIVIOS and Vertebral Spacer-PR concept. The open structure and the interface to the implant holder is the same. In order to ease the rotation of the implant, one row of teeth has been removed and is replaced by a chamfer. The cross-section is adapted to the Contact Fusion Cage sizes of 7 8 mm, 9 8 mm, 10 8 mm, 11 9 mm, 12 10 mm, 13 10 mm, and 15 11 mm. The implants provide two lordotic angles of 4 and 8. All implants are available empty or prefilled with chronOS block material. PLIVIOS provides benefits in dorsal arthrodesis with cage by preserving the integrity of the endplates and their stability.
The Vertebral Spacer PR is a radiolucent spacer system designed for use in interbody procedures for L1 through L5. The footprint is 8 x 22 mm and comes in 7, 9, 11, 13, 15, and 17 mm heights. This narrow width allows for a smaller window to be created, by a partial laminectomy, lateral to the spinal cord without compromising the facet joints and structural stability of that vertebral level.
The spacer is made out of PEEK Optima, a radiolucent, biocompatible, inert polymer material. It has a rectangular footprint and convex cranial/caudal surfaces to match the vertebral endplate geometry. The implant has a scaffold-like structure which reduces the overall amount of synthetic material needed. A central hole allows the use of allograft. The Vertebral Spacer PR contains two radio-opaque pins for proper placement under fluoroscopy and recognition in follow-up evaluations.
New instruments for PLIVIOS
Implant Holder for PLIVIOS
The Implant Holder has a strengthened shaft with a keel which avoids the shearing-off of the instrument. The shaft elements are rounded in order to avoid mechanical stress to the surrounding tissue while rotating the instrument. The transition of the tips to the shaft is rounded in order to reduce the risk of breaking.
The Nerve Root Retractor and Packing Block
The Nerve Root Retractor is a new protection instrument which allows a safer access to the intervertebral space through the posterior access. The frontal blades are inserted between the nerve root and the dura. The metal blades protect the mentioned neural structure from major damages, eventually caused from the toothed implant.
The Packing Block comprises four cavities for the individual width of the implants.
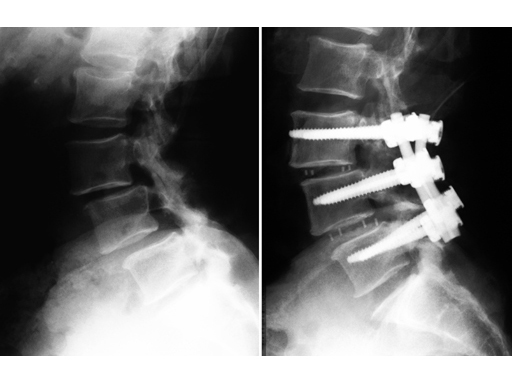
64-year-old woman, lateral, pre- and postoperative x-rays.
Hazards and labeling
Due to varying countries’ legal and regulatory approval requirements, consult the appropriate local product labeling for approved intended use of the products described on this website. All devices on this website are approved by the AO Technical Commission. For logistical reasons, these devices may not be available in all countries worldwide at the date of publication.
Legal restrictions
This work was produced by AO Foundation, Switzerland. All rights reserved by AO Foundation. This publication, including all parts thereof, is legally protected by copyright.
Any use, exploitation or commercialization outside the narrow limits set forth by copyright legislation and the restrictions on use laid out below, without the publisher‘s consent, is illegal and liable to prosecution. This applies in particular to photostat reproduction, copying, scanning or duplication of any kind, translation, preparation of microfilms, electronic data processing, and storage such as making this publication available on Intranet or Internet.
Some of the products, names, instruments, treatments, logos, designs, etc referred to in this publication are also protected by patents, trademarks or by other intellectual property protection laws (eg, “AO” and the AO logo are subject to trademark applications/registrations) even though specific reference to this fact is not always made in the text. Therefore, the appearance of a name, instrument, etc without designation as proprietary is not to be construed as a representation by the publisher that it is in the public domain.
Restrictions on use: The rightful owner of an authorized copy of this work may use it for educational and research purposes only. Single images or illustrations may be copied for research or educational purposes only. The images or illustrations may not be altered in any way and need to carry the following statement of origin “Copyright by AO Foundation, Switzerland”.
Check www.aofoundation.org/disclaimer for more information.
If you have any comments or questions on the articles or the new devices, please do not hesitate to contact us.
“approved by AO Technical Commission” and “approved by AO”
The brands and labels “approved by AO Technical Commission” and “approved by AO”, particularly "AO" and the AO logo, are AO Foundation's intellectual property and subject to trademark applications and registrations, respectively. The use of these brands and labels is regulated by licensing agreements between AO Foundation and the producers of innovation products obliged to use such labels to declare the products as AO Technical Commission or AO Foundation approved solutions. Any unauthorized or inadequate use of these trademarks may be subject to legal action.
AO ITC Innovations Magazine
Find all issues of the AO ITC Innovations Magazine for download here.
Innovation Awards
Recognizing outstanding achievements in development and fostering excellence in surgical innovation.