Cannulated Headless Screw (Fully Threaded) System
Tom Fischer, Sudhir Warrier, Alex Lluch, Martin Langer, Stephen Tham
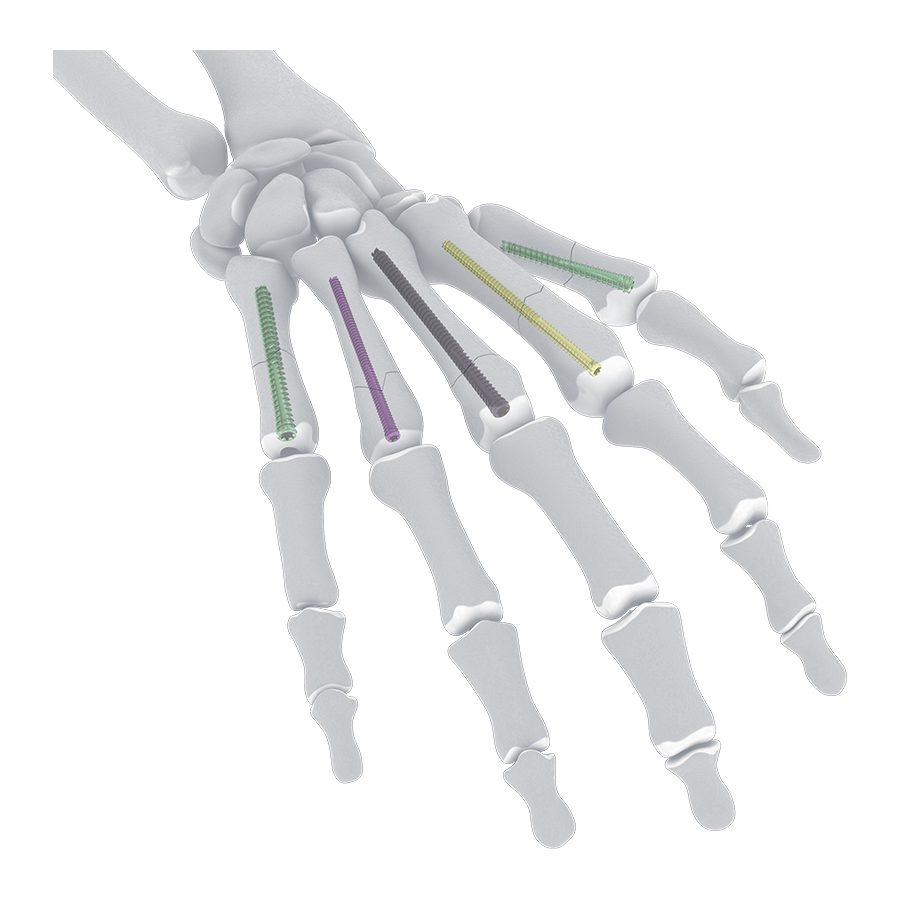
Developed by members of the Hand Expert Group and approved by the AO Technical Commission in September 2025, the Cannulated Headless Screw (Fully Threaded) System (Fig 1) is indicated for use in bone reconstruction, osteotomy, arthrodesis, joint fusion, fracture repair, and fracture fixation of bones appropriate for the size of the device. The initial product launch in 2025 will focus on hand and wrist procedures (screw diameters 2.0-4.5 mm).
The new system was developed as a line extension to the Cannulated Compression Headless Screw (CCHS) System, approved in 2019 with indications for the foot and ankle. The use of cannulated screws follows the principles of interfragmentary screw fixation and aims to optimize screw position.
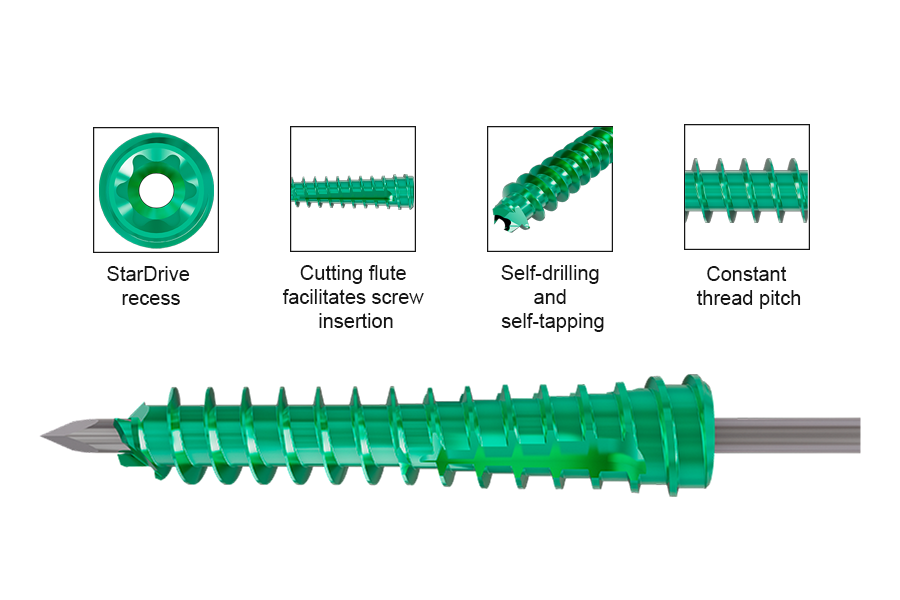
The new screw design incorporates a patented self-tapping, self-drilling screw tip that allows for precise placement. The screw cannulation feature allows placement over a guidewire to facilitate better accuracy before drilling or screw insertion (Fig 2). Containing a comprehensive range of implants, the system is engineered to meet the needs of patients with diverse anatomy (see Details tab below).
System overview
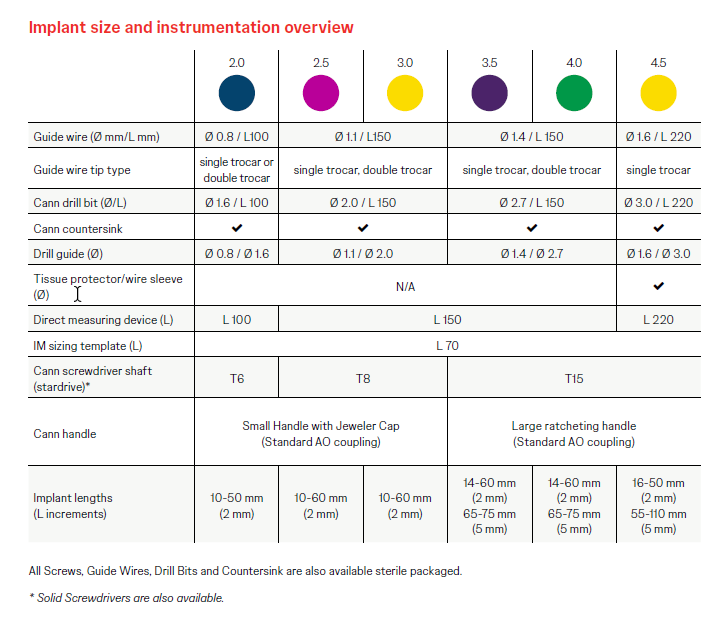
The Cannulated Headless Screw (Fully Threaded) features a constant thread pitch and is fully threaded with a non-compressive screw design. With self-drilling and self-tapping elements (Fig 2), the cutting tip design is identical to that of CCHS. Screws are available in a range of diameters (2.0, 2.5, 3.0, 3.5, 4.0, 4.5 mm) and in lengths from 10 mm – 110 mm (Fig 3). Screws are manufactured in titanium alloy (Ti-6Al-4V ELI) and are cannulated for use with a guide wire.
For ease of handling, the screws are color-coded to the instrumentation in a schema identical to that of CCHS (Fig 3). The new system is available in sterile and non-sterile implant and instrument packaging options and is provided in modular sets. A significant advantage for hospital inventory management is the similar geometry of the new screws and CCHS, offering compatibility with CCHS screw racks, graphic cases, and instrumentation.
Features and Benefits of the new Cannulated Headless Screw (Fully Threaded) System
- Unique angled cutting tip: increased sharpness at the screw tip may reduce surgeon effort to achieve screw insertion, allowing better final screw alignment.
- Stiffer Guidewire (CoCr): may reduce guidewire deflection during screw insertion, allowing the surgeon to better judge screw depth and trajectory, and ultimately to place the screw more accurately.
- Comprehensive options: the extensive range of screw diameters and lengths in the new Cannulated Headless Screw (Fully Threaded) System addresses the complexity and unforeseen challenges in surgical procedures arising from varying patient anatomy.
- Broadened portfolio: previously, only cannulated compression headless screws were offered. This line extension introduces fully threaded, constant pitch headless screws that are non-compressive.
Indications in EU and US
The Cannulated Headless Screw (Fully Threaded) System is indicated for use in bone reconstruction, osteotomy, arthrodesis, joint fusion, fracture repair, and fracture fixation of bones appropriate for the size of the device. Screws are intended for single use only.
Contraindications
The implant should not be used in a patient who has current, or who has a history of:
- Local or systemic acute or chronic inflammation;
- Active infection or inflammation;
- Suspected or documented metal allergy or intolerance
Hazards and labeling
Due to varying countries’ legal and regulatory approval requirements, consult the appropriate local product labeling for approved intended use of the products described on this website. All devices on this website are approved by the AO Technical Commission. For logistical reasons, these devices may not be available in all countries worldwide at the date of publication.
Legal restrictions
This work was produced by AO Foundation, Switzerland. All rights reserved by AO Foundation. This publication, including all parts thereof, is legally protected by copyright.
Any use, exploitation or commercialization outside the narrow limits set forth by copyright legislation and the restrictions on use laid out below, without the publisher‘s consent, is illegal and liable to prosecution. This applies in particular to photostat reproduction, copying, scanning or duplication of any kind, translation, preparation of microfilms, electronic data processing, and storage such as making this publication available on Intranet or Internet.
Some of the products, names, instruments, treatments, logos, designs, etc referred to in this publication are also protected by patents, trademarks or by other intellectual property protection laws (eg, “AO” and the AO logo are subject to trademark applications/registrations) even though specific reference to this fact is not always made in the text. Therefore, the appearance of a name, instrument, etc without designation as proprietary is not to be construed as a representation by the publisher that it is in the public domain.
Restrictions on use: The rightful owner of an authorized copy of this work may use it for educational and research purposes only. Single images or illustrations may be copied for research or educational purposes only. The images or illustrations may not be altered in any way and need to carry the following statement of origin “Copyright by AO Foundation, Switzerland”.
Check www.aofoundation.org/disclaimer for more information.
If you have any comments or questions on the articles or the new devices, please do not hesitate to contact us.
“approved by AO Technical Commission” and “approved by AO”
The brands and labels “approved by AO Technical Commission” and “approved by AO”, particularly "AO" and the AO logo, are AO Foundation's intellectual property and subject to trademark applications and registrations, respectively. The use of these brands and labels is regulated by licensing agreements between AO Foundation and the producers of innovation products obliged to use such labels to declare the products as AO Technical Commission or AO Foundation approved solutions. Any unauthorized or inadequate use of these trademarks may be subject to legal action.
AO ITC Innovations Magazine
Find all issues of the AO ITC Innovations Magazine for download here.
Innovation Awards
Recognizing outstanding achievements in development and fostering excellence in surgical innovation.