CMORE® Cervicothoracic System
Christian Mazel, Lorin Benneker, Michelle Clarke, Naresh Kumar, Firoz Miyanji, Yu-Mi Ryang, Maarten Spruit, Jean-Paul Wolinsky, Richard Bransford
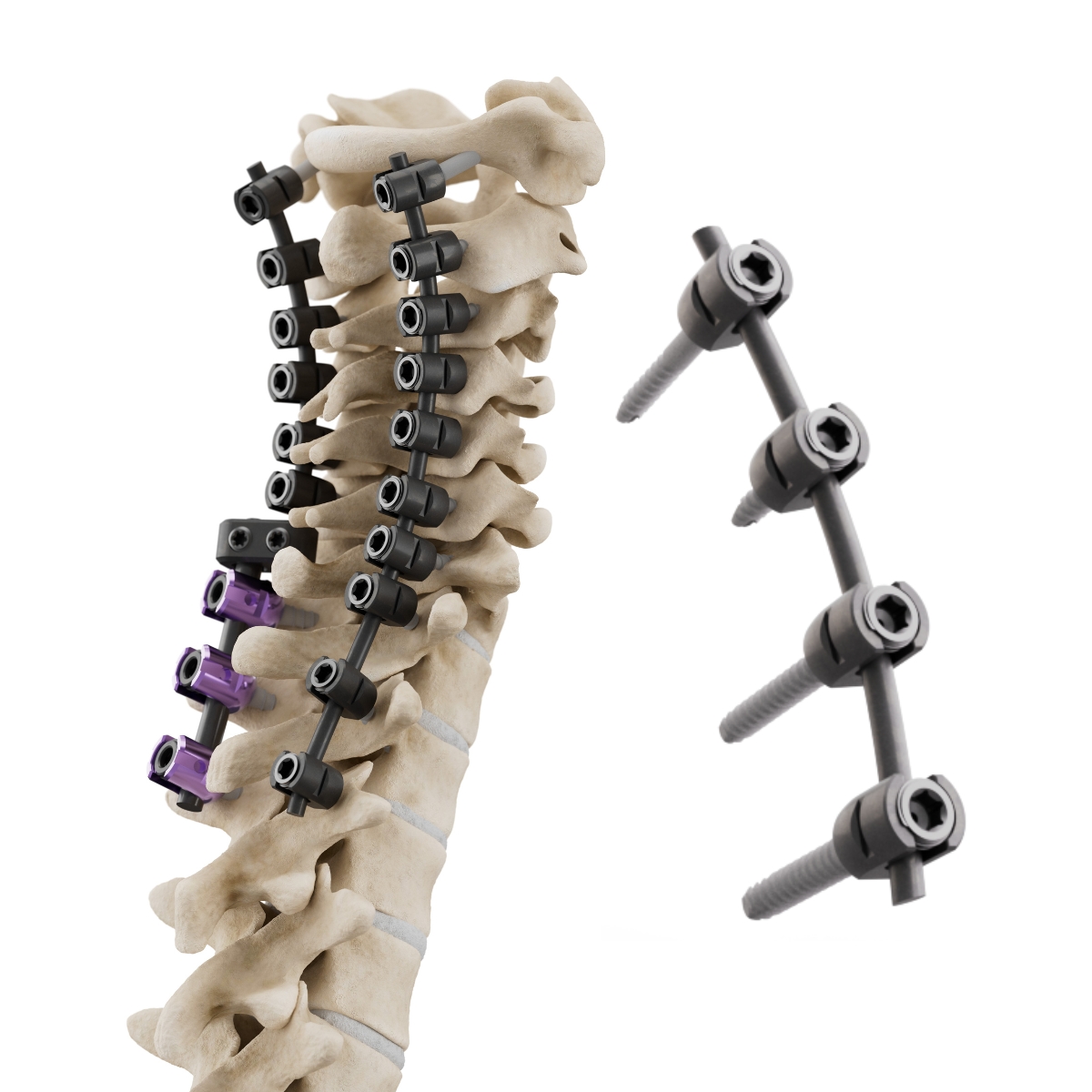
Approved by the AO Technical Commission in December 2025, the CMORE® Cervicothoracic System (Fig 1) is a novel spine instrumentation system that is BlackArmor® engineered Carbon/PEEK. Intended for use in patients with spinal tumors, de-novo spinal infections, and additional indications, the system offers a radiolucent alternative to conventional metallic implants. The clinical advantages of BlackArmor® for patients and surgical teams include more precise targeting of radiation treatment, superior local tumor control, earlier detection of tumor recurrence and compatibility with advanced radiation modalities.
The product portfolio includes:
- Screws in a different range of diameters and lengths
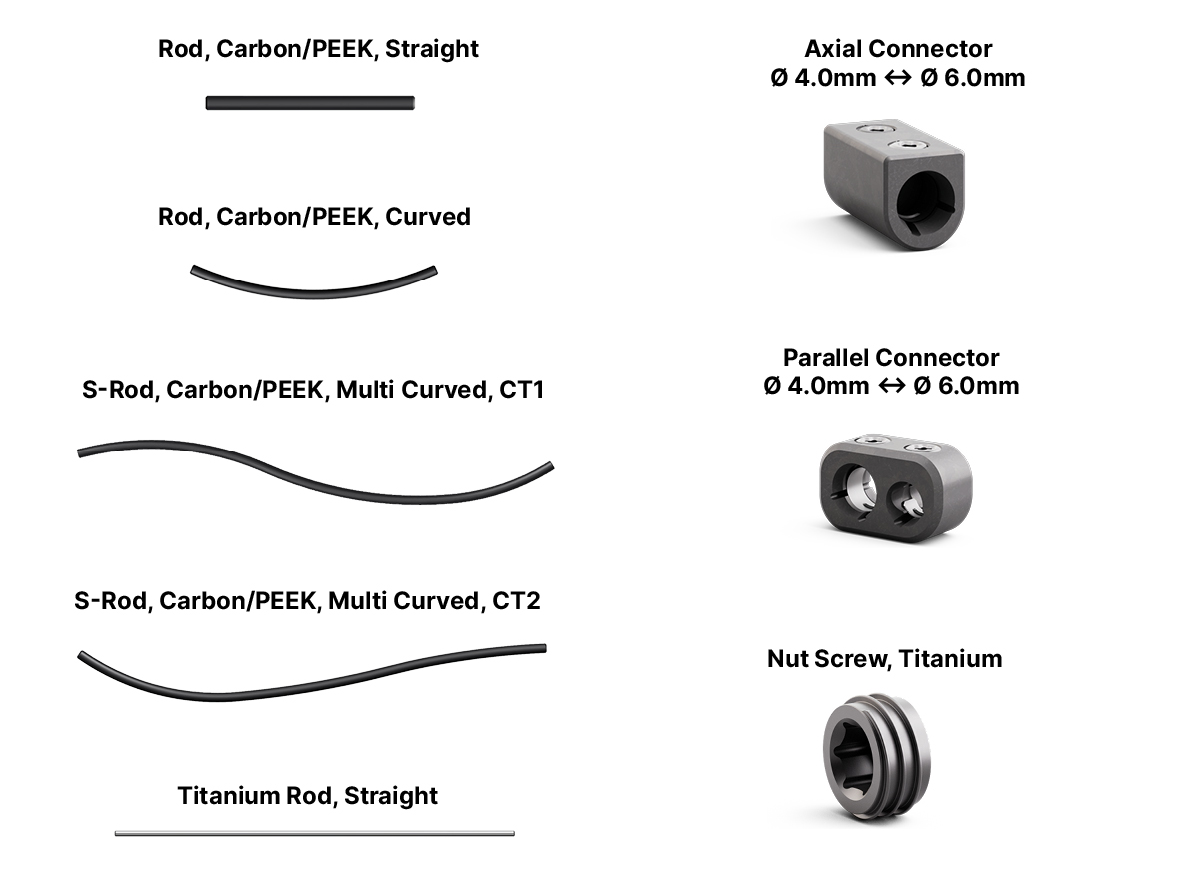
- BlackArmor® engineered Carbon/PEEK rods in various lengths and curvatures
- Titanium straight rods
- Axial and parallel connectors
CMORE® Pedicle Screw
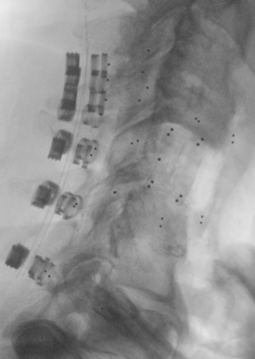
The CMORE® Screw features a BlackArmor® tulip, a titanium nut screw, and tantalum markers, allowing the implant a degree of imaging visibility (Fig 2 and Fig 4). The screws are available in 4.0 mm, 4.5 mm and 5.0 mm diameters. The shank screw is available in a diameter of 4.0 mm.
The CMORE® CT System is the first spinal implant that can also serve as a fiducial marker in radiotherapy [1]. It uses novel tantalum markers for IMR tracking with submillimeter accuracy, and provides greater confidence in the precision and safety of treatment while minimizing dose error to the target and spinal cord. This could potentially reduce both recurrence and toxicity to the patient.
CMORE® Rods and Connectors
The CMORE® CT System offers a comprehensive selection of BlackArmor® Carbon/PEEK rods (4.0 mm diameter, maximum length 200 mm), available in straight, curved, and S-configurations (Fig 3). Straight titanium rods supplement the system with a maximum length of 300 mm.
The connectors allow the secure joining of rods from the CMORE® CT and VADER® Pedicle Systems (Fig 3). Design features include titanium screws and a titanium ring that allows flexibility for rod insertion.
The CMORE® Parallel- and Axial Connector can accommodate 4.0 mm Carbon/PEEK or 4.0 mm titanium rods and link them to 6.0 mm Carbon/PEEK or 6.0 mm titanium rods.
Radiolucency and Clinical Benefits of BlackArmor® Carbon/PEEK implants
The radiolucency of BlackArmor® engineered Carbon/PEEK (Fig 4) offers numerous clinical benefits for spinal tumor patients:
More Precise Radiation Treatment
- BlackArmor® implants offer significant reduction in metal artifacts compared to titanium, improving postoperative imaging accuracy [2].
- Increased accuracy in target and delineation of organs at risk (OAR), optimal radiation plan quality, and unimpeded dose delivery [2].
Improved Local Tumor Control
- BlackArmor® implants allow the delivery of a higher radiation dose without scattering (cold spots), reducing infield or marginal recurrence [3].
Earlier Recurrence Detection
- In a matched cohort study (99 CFR-PEEK vs. 49 titanium cases), tumor recurrence was detected one scan earlier with Carbon/PEEK (mean94days vs.189days; p=0.013) [4].
Advanced Radiation Modalities
- BlackArmor® implants enable accurate proton and stereotactic photon planning with minimal dose uncertainty. The target coverage and OAR sparing is equivalent to that of non-instrumented spine [5].
References
- Lee-Poprocki H, Ritter AR, Upadhyay R, et al. Novel Intrafraction Motion Tracking During Postoperative Spine Stereotactic Irradiation for a Patient With Carbon Fiber Fixation Hardware. Practical Radiation Oncology. (2023) Nov-Dec;13 (6): 510-516.
- de Almeida R, Ghia A, Amini B et al. Quantification of MRI Artifacts in Carbon Fiber Reinforced Polyetheretherketone Thoracolumbar Pedicle Screw Constructs prior to Spinal Stereotactic Radiosurgery. Practical Radiation Oncology. (2024); 14 (2): 103 –111.
- de Almeida R, Call-Orellana F, Zuluaga-Garcia J et al. Local Control After Adjuvant Radiosurgery for Spinal Metastasis Treated With Decompression and Posterior Segmental Stabilization: A Comparison Between Carbon Fiber/Polyetheretherketone-Based and Metallic Implants. Advances in Radiation Oncology. (2025); 10 (7): 101806.
- Ward J, Damante M, Wilson S et al. Impact of instrumentation material on local recurrence: a case-matched series using carbon fiber-PEEK vs. titanium. Journal of Neuro-Oncology. (2025); 171: 155–162.
- Henzen D, Schmidhalter D, Guyer G et al. Feasibility of postoperative spine stereotactic body radiation therapy in proximity of carbon and titanium hybrid implants using a robotic radiotherapy device. Radiation Oncology. (2022); 17, 94.
Hazards and labeling
Due to varying countries’ legal and regulatory approval requirements, consult the appropriate local product labeling for approved intended use of the products described on this website. All devices on this website are approved by the AO Technical Commission. For logistical reasons, these devices may not be available in all countries worldwide at the date of publication.
Legal restrictions
This work was produced by AO Foundation, Switzerland. All rights reserved by AO Foundation. This publication, including all parts thereof, is legally protected by copyright.
Any use, exploitation or commercialization outside the narrow limits set forth by copyright legislation and the restrictions on use laid out below, without the publisher‘s consent, is illegal and liable to prosecution. This applies in particular to photostat reproduction, copying, scanning or duplication of any kind, translation, preparation of microfilms, electronic data processing, and storage such as making this publication available on Intranet or Internet.
Some of the products, names, instruments, treatments, logos, designs, etc referred to in this publication are also protected by patents, trademarks or by other intellectual property protection laws (eg, “AO” and the AO logo are subject to trademark applications/registrations) even though specific reference to this fact is not always made in the text. Therefore, the appearance of a name, instrument, etc without designation as proprietary is not to be construed as a representation by the publisher that it is in the public domain.
Restrictions on use: The rightful owner of an authorized copy of this work may use it for educational and research purposes only. Single images or illustrations may be copied for research or educational purposes only. The images or illustrations may not be altered in any way and need to carry the following statement of origin “Copyright by AO Foundation, Switzerland”.
Check www.aofoundation.org/disclaimer for more information.
If you have any comments or questions on the articles or the new devices, please do not hesitate to contact us.
“approved by AO Technical Commission” and “approved by AO”
The brands and labels “approved by AO Technical Commission” and “approved by AO”, particularly "AO" and the AO logo, are AO Foundation's intellectual property and subject to trademark applications and registrations, respectively. The use of these brands and labels is regulated by licensing agreements between AO Foundation and the producers of innovation products obliged to use such labels to declare the products as AO Technical Commission or AO Foundation approved solutions. Any unauthorized or inadequate use of these trademarks may be subject to legal action.
AO ITC Innovations Magazine
Find all issues of the AO ITC Innovations Magazine for download here.
Innovation Awards
Recognizing outstanding achievements in development and fostering excellence in surgical innovation.