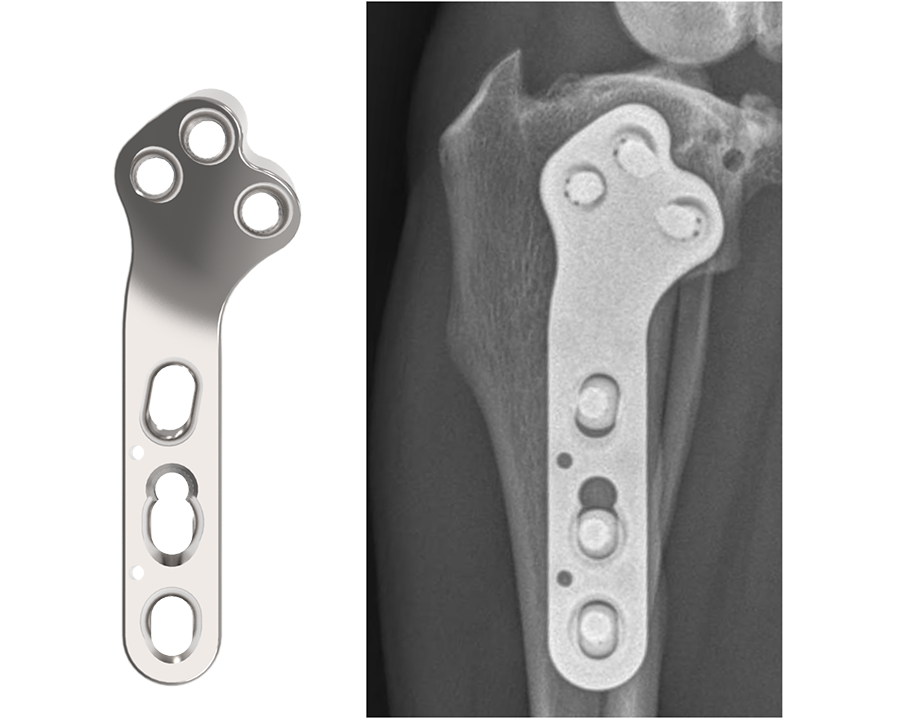
3.5 mm TPLO Plate with Advanced Radial Compression (ARC)
Michael Kowaleski, William B Saunders, Erik Asimus, Alexis Bilmont, Loïc Déjardin
The DePuy Synthes TPLO System is meticulously designed for stabilizing osteotomies of the canine proximal tibia, ensuring stable fixation and reliable healing. Developed in collaboration with and approved by the AO Technical Commission, the ARC TPLO plate comprises two 3.5 mm plates, for both left and right applications.
This innovative system is engineered to deliver precise compression across the osteotomy, leveraging specialized compression holes along the shaft. This targeted compression not only fosters direct bone healing but also bolsters resistance against potential rock-back failures. Moreover, it streamlines the contour of the proximal plate and optimizes screw placements, reducing the necessity for plate contouring while ensuring dependable locking screw positioning. The design features a consistent locking interface between the plate and the locking screws, reinforcing stability and integrity.
The accompanying array of specialized instruments, including drill guides, TPLO jigs, saw guides, and saw blades, facilitates surgical execution, versatile implant positioning, pinpoint accuracy in osteotomy location, and guided stability of the saw blade.
The ARC TPLO plate stands out for its continuous, precisely directed compression across the osteotomy site. Notably, the proximal section of the ARC TPLO plate is angled in a slightly more caudal direction to accommodate diverse patient anatomies. Furthermore, the trajectories of proximal locking screws are designed to steer clear of the articular surface while engaging the central mass of the proximal tibia, ensuring optimal fixation and stability.
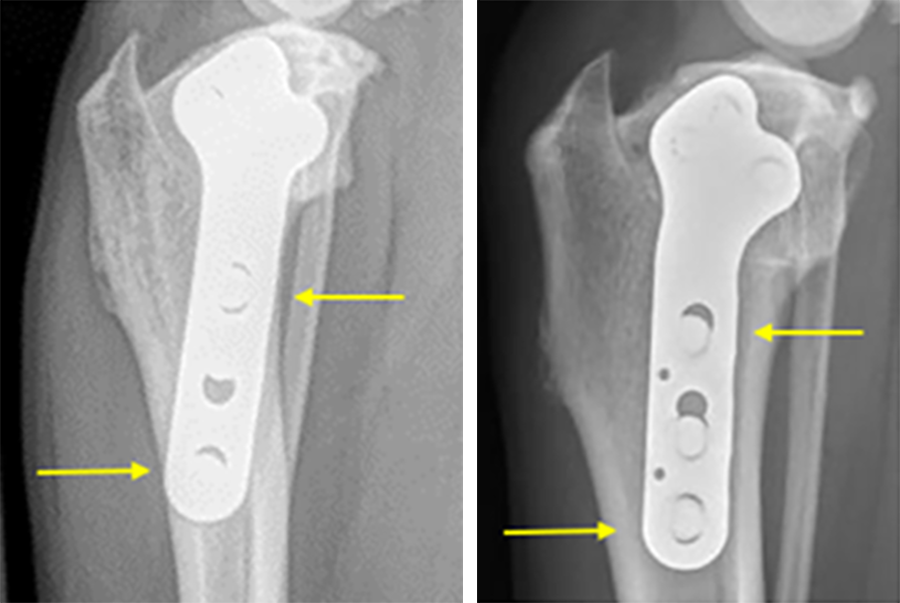
The currently available DePuy Synthes 3.5 mm TPLO plate is generally applied at a slight angle relative to the mechanical axis of the tibia. While this has proven to be an ideal angle from a compression standpoint, there may be instances where the distal‐most screw is close to the cranial cortex of the tibia and the third most distal screw is close to the caudal cortex. In extreme cases these screw locations could cause increased bone stress in those regions.
The newly innovated implant, 3.5 mm TPLO with Advanced Radial Compression (ARC), locates these screws closer to the center of the bone while preserving the clinically‐proven angle of compression.
TPLO ARC Technology
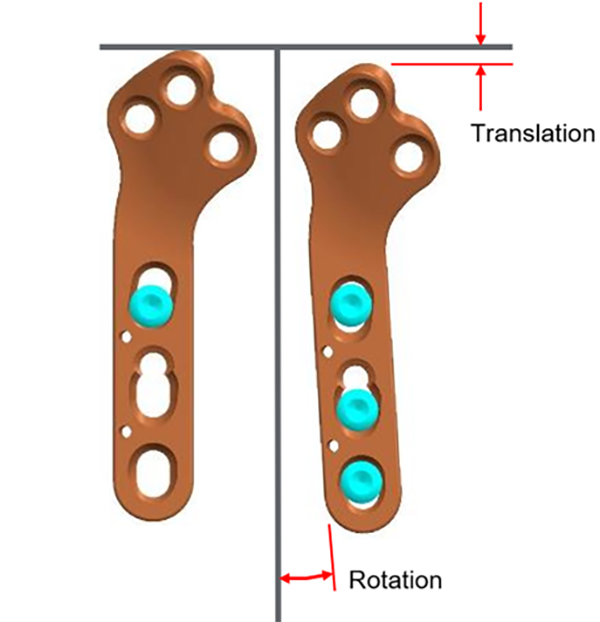
The ARC TPLO plate provides continuous, controlled, and directed compression across the osteotomy via a unique system of holes that serves to translate and rotate the plate simultaneously during compression. The resultant direction of compression has been designed to match that of the DPS standard TPLO plate, which is clinically proven to provide excellent healing and improved resistance to rock‐back.
Additionally, the proximal section of the ARC TPLO plate is more caudally oriented to provide better anatomical fit on some patients.
And, as with all DPS TPLO plates, the proximal locking screw trajectories are designed to avoid the articular surface and engage the central mass of the proximal tibia.
System content and description
The ARC TPLO plate comprises two 3.5 mm plates, for both left and right applications.
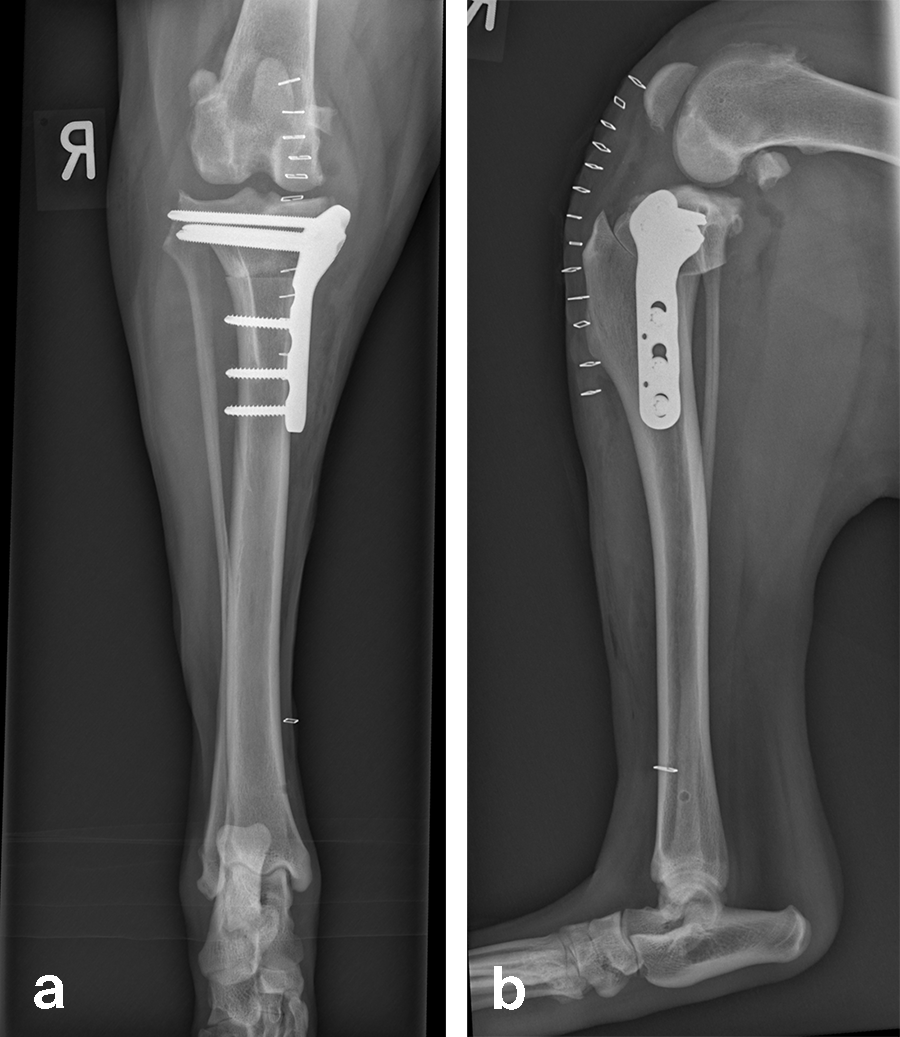
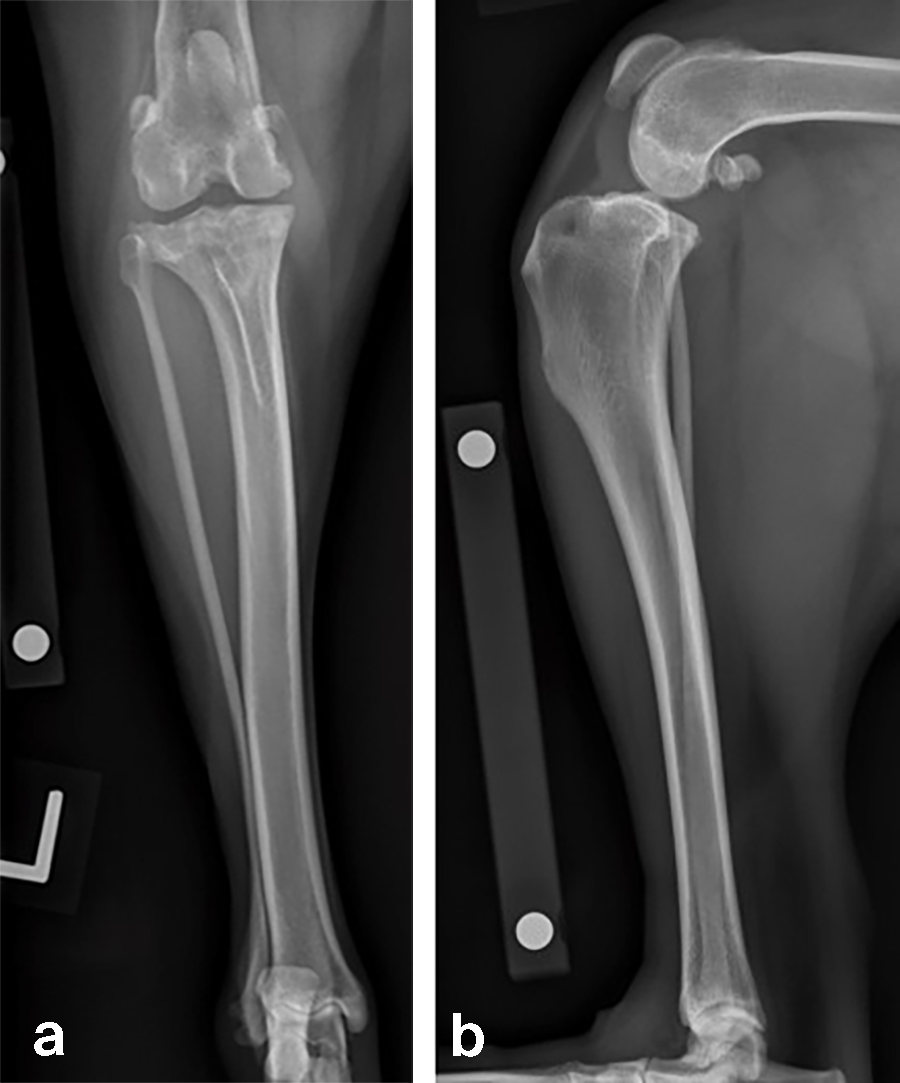
Case 1: Bentley Hoe, male neutered Golden Retriever, 32 kg
(Case provided by Alexis Bilmont, West Midlands, England)
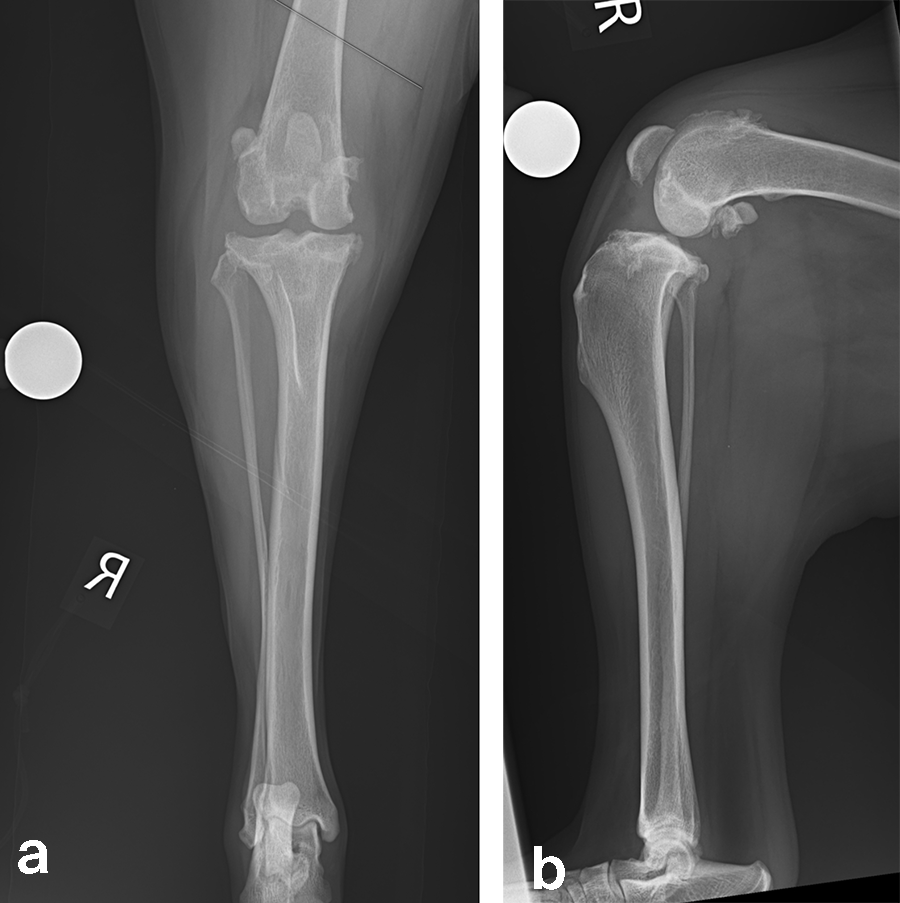
A 32 kg male neutered Golden Retriever presented with a recent deterioration of chronic right hind limb lameness. Physical examination revealed a cranial cruciate ligament rupture.
Follow-up x-rays at 8 weeks postoperatively revealed stable implants, stable bone segments, and healing of the osteotomy. The clinical outcome was satisfactory.
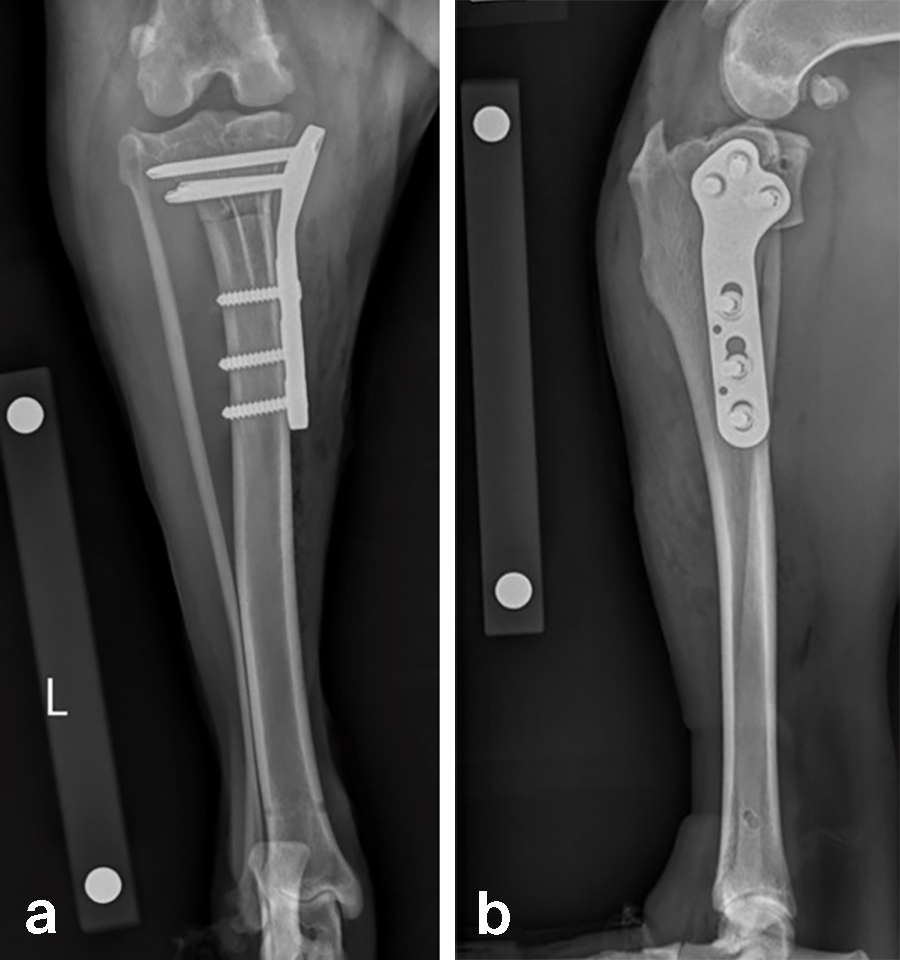
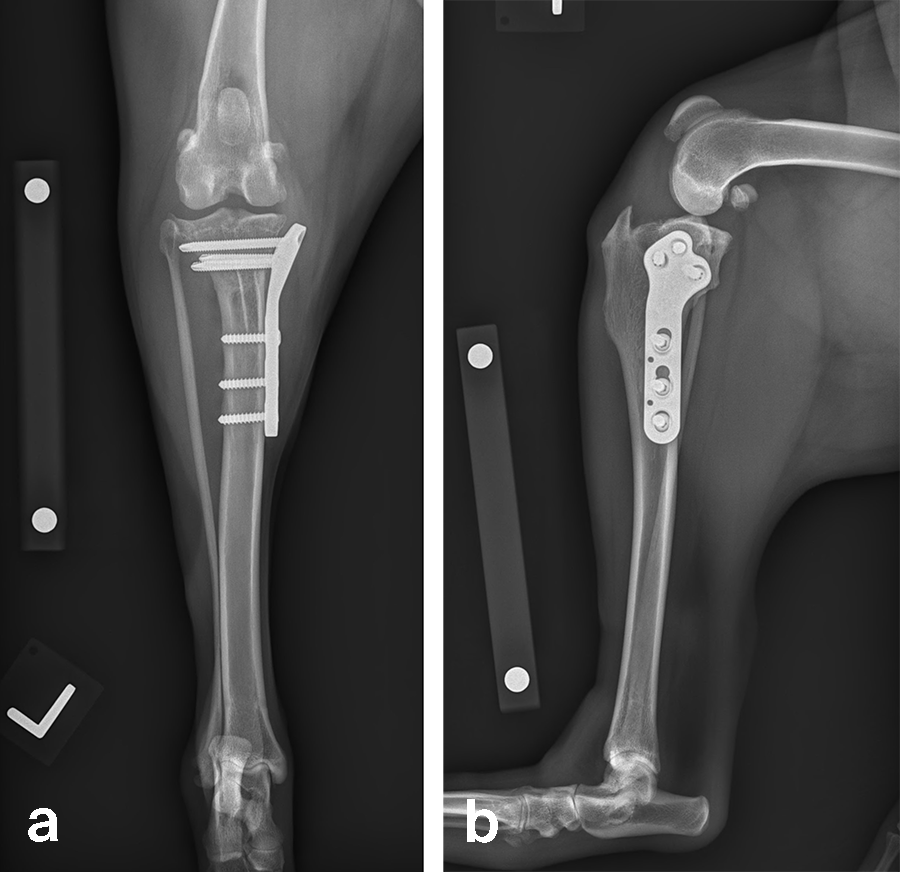
Case 2: Rosie, 3‐year‐old black Labrador Retriever, 23.3 kg
(Case provided by William B Saunders, Texas, USA)
A 23.3 kg 3-year-old spayed female black Labrador Retriever presented with a history of chronic, progressive left pelvic limb lameness. Her clinical examination was suggestive of left cranial cruciate ligament (CCL) rupture. Medical and surgical treatment options were discussed with the clients. They elected to have Rosie’s knee treated with arthroscopy and TPLO.
Left stifle arthroscopy was performed and a partial CCL rupture with incompetent remand was identified. The remaining CCL was debrided with a motorized shaver. The meniscus was healthy/non-injured based on visual inspection and probing. Arthroscopy portals were closed, and exposure of the proximomedial tibia was performed.
Based on preoperative templating, a 21 mm radial saw blade was used to perform an osteotomy of the proximal tibia. The plateau was leveled to a final target slope of 5° and the osteotomy was stabilized with a 3.5 mm ARC TPLO plate.
In Rosie’s case, the plate fit was excellent and the TPLO was performed without complication. Postoperative radiographs illustrate excellent plate position and screw placement and compression across all aspects of the osteotomy. Rosie recovered uneventfully from surgery and at the time of recheck (7 weeks postoperation) was using the operated limb without visible lameness. The knee examination was unremarkable, and radiographs demonstrated robust healing of the TPLO.
Hazards and labeling
Due to varying countries’ legal and regulatory approval requirements, consult the appropriate local product labeling for approved intended use of the products described on this website. All devices on this website are approved by the AO Technical Commission. For logistical reasons, these devices may not be available in all countries worldwide at the date of publication.
Legal restrictions
This work was produced by AO Foundation, Switzerland. All rights reserved by AO Foundation. This publication, including all parts thereof, is legally protected by copyright.
Any use, exploitation or commercialization outside the narrow limits set forth by copyright legislation and the restrictions on use laid out below, without the publisher‘s consent, is illegal and liable to prosecution. This applies in particular to photostat reproduction, copying, scanning or duplication of any kind, translation, preparation of microfilms, electronic data processing, and storage such as making this publication available on Intranet or Internet.
Some of the products, names, instruments, treatments, logos, designs, etc referred to in this publication are also protected by patents, trademarks or by other intellectual property protection laws (eg, “AO” and the AO logo are subject to trademark applications/registrations) even though specific reference to this fact is not always made in the text. Therefore, the appearance of a name, instrument, etc without designation as proprietary is not to be construed as a representation by the publisher that it is in the public domain.
Restrictions on use: The rightful owner of an authorized copy of this work may use it for educational and research purposes only. Single images or illustrations may be copied for research or educational purposes only. The images or illustrations may not be altered in any way and need to carry the following statement of origin “Copyright by AO Foundation, Switzerland”.
Check www.aofoundation.org/disclaimer for more information.
If you have any comments or questions on the articles or the new devices, please do not hesitate to contact us.
“approved by AO Technical Commission” and “approved by AO”
The brands and labels “approved by AO Technical Commission” and “approved by AO”, particularly "AO" and the AO logo, are AO Foundation's intellectual property and subject to trademark applications and registrations, respectively. The use of these brands and labels is regulated by licensing agreements between AO Foundation and the producers of innovation products obliged to use such labels to declare the products as AO Technical Commission or AO Foundation approved solutions. Any unauthorized or inadequate use of these trademarks may be subject to legal action.
AO ITC Innovations Magazine
Find all issues of the AO ITC Innovations Magazine for download here.
Innovation Awards
Recognizing outstanding achievements in development and fostering excellence in surgical innovation.