ALTALYNE Ultra Alignment System
Christian Mazel, Firoz Miyanji
Scoliosis is an abnormal curvature of the spine, and adolescent idiopathic scoliosis (AIS) is the most common spinal deformity in the pediatric population, with a worldwide prevalence ranging from 0.47% to 12% [1−7]. Surgical treatment of AIS traditionally involves 3D correction with rods and fusion of the spine.
Intraoperative rod flattening is a common challenge faced by surgeons which often results in failed kyphosis restoration and a suboptimal postoperative sagittal profile.
The ALTALYNE Ultra Alignment System is designed to specifically address the challenges of intraoperative rod flattening and its high bending yield strength helps maintain its rod contour to restore the ideal thoracic kyphosis for patients.
Background
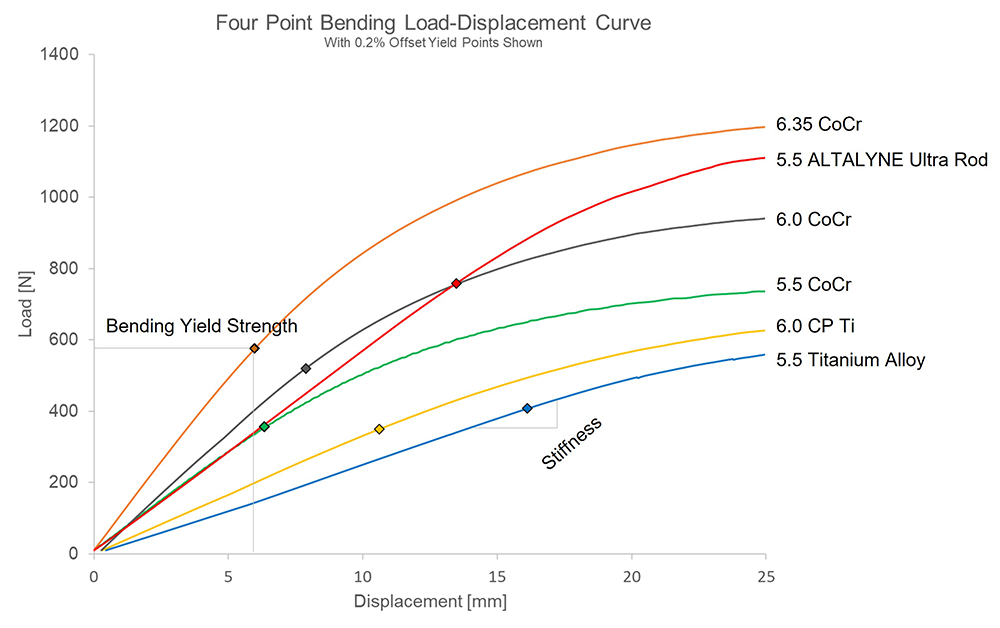
Restoration of ideal thoracic kyphosis is a significant challenge in adolescent spinal deformities, and in particular AIS because of its inherent thoracic lordotic sagittal profile. Rods with a higher bending yield strength are more resistant to intraoperative flattening, which means the rods can maintain their customized contour and help surgeons achieve the desired sagittal restoration for their patients [8]. With Ti and traditional CoCr alloys, surgeons tend to select larger diameter rods (6.0 mm) to increase the bending yield strength of the rod and hence sacrifice on profile.
The solution
Through advanced material science using a special Cobalt Chromium alloy (CoNiCrMo), ALTALYNE Ultra 5.5 mm rods provide improved bending yield strength over existing 6.0 mm CoCr rods [9]. The higher bending yield strength may result in less rod flattening compared with existing rods with the added advantage of being lower profile.
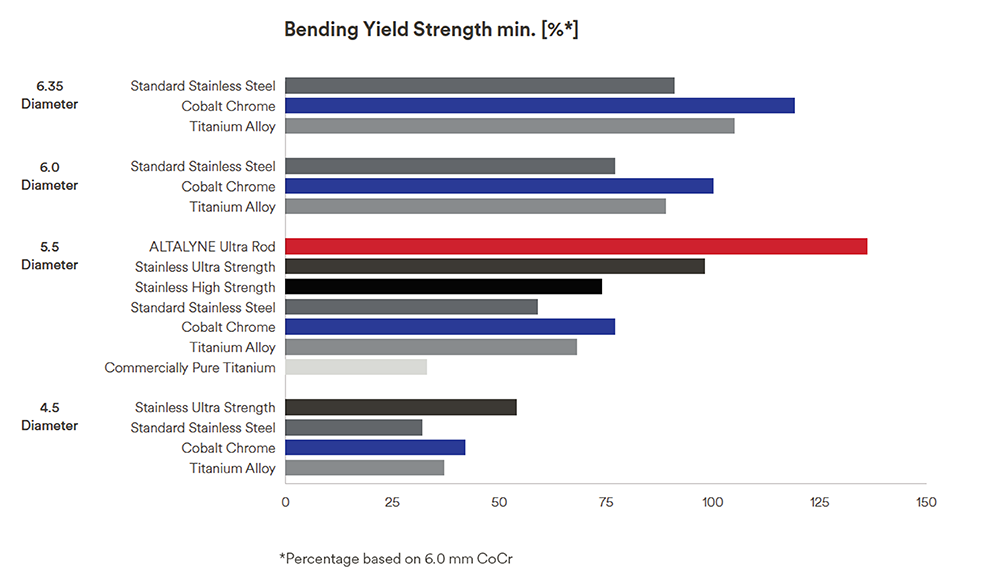
The forces required to bend rods of different materials and diameters are illustrated in Fig 1. The slope of the curve indicates the stiffness of the rod. The points at which the rod permanently deforms, or bends is the yield strength. Fig 1 shows that ALTALYNE Ultra Rods have a higher bending yield strength compared with other rods. The bending yield strength is 36% higher compared to 6.0 mm CoCr rods and 47% higher compared to 6.0 mm Ti rods (Fig 2) [9].
System overview
The ALTALYNE Ultra Alignment System includes straight and precontoured rods, and the ALTALYNE Ultra French Rod Bender and Tabletop Cutter. Rod Templates are available for estimating rod shape/length.
Rods
The ALTALYNE Ultra Rods are compatible with EXPEDIUM™ 5.5 Spine System and EXPEDIUM VERSE Spine System.
They are available in straight lengths from 120 mm to 600 mm and precontoured (S, M, L, XL) (Fig 3). Clinical literature indicates that rod fractures often occur at notches introduced at the intraoperative bending sites [10]. The ALTALYNE Ultra Rod precontoured configurations require less manual bending than straight rods and reduce notching done intraoperatively.
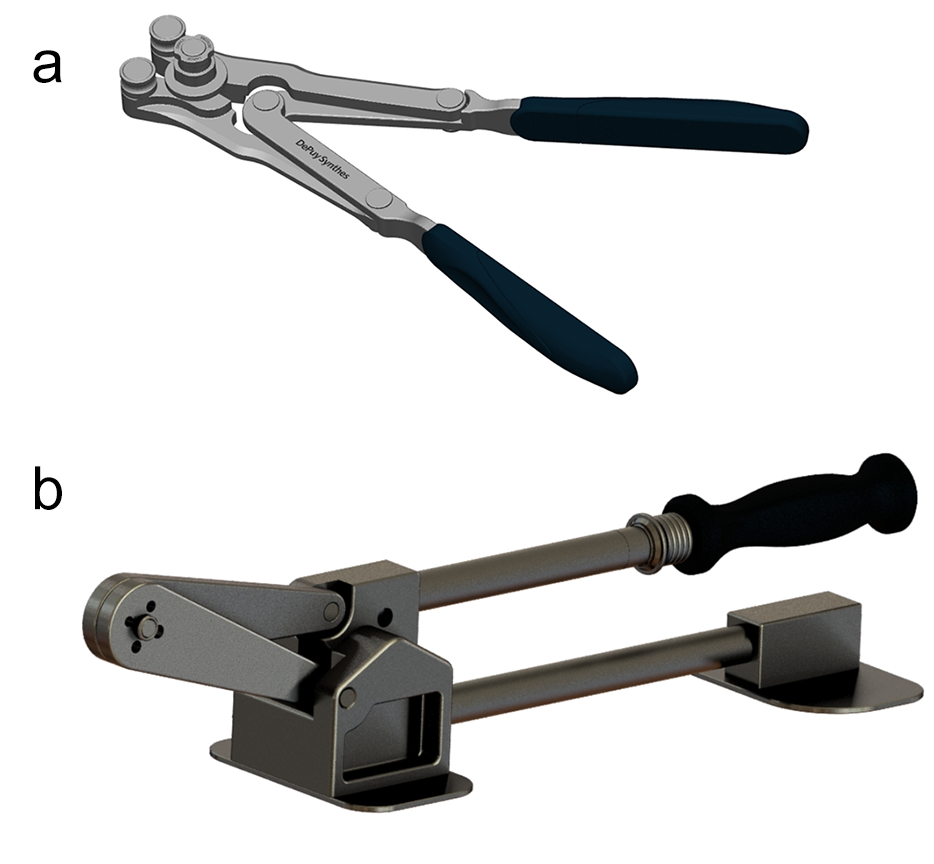
French Rod Bender and Tabletop Cutter
The force required to cut and contour rods may depend on the rod material selected. In-situ bending is more challenging with CoNiCrMo alloy rods.
To bend and cut ALTALYNE Ultra Rods the instrumentation has been updated, and a dedicated Dual Action French Rod Bender and Tabletop Cutter have been developed (Fig 4). Both instruments have a mechanical advantage over the EXPEDIUM VERSE French Rod Bender (+46%) and the EXPEDIUM Spine System Cutter (+38%) [11, 12].
Conclusion
ALTALYNE Ultra Alignment system provides surgeons with a new treatment option through technologically advanced materials and instrumentation to deliver greater bending yield strength than traditional rods, and most importantly, the potential for better patient outcomes. The system is expected to help achieve and maintain optimum thoracic kyphosis and improve surgical outcomes in the treatment of adolescent spinal deformities, particularly AIS.
It is important to follow the surgical techniques of EXPEDIUM 5.5 Spine System or EXPEDIUM VERSE Spine System with the ALTALYNE Ultra Rods. Knowledge about different mechanical properties of different rod materials (Ti, SS, CoCr, ALTALYNE Ultra) and their diameters is mandatory for deciding which rod is appropriate.
Limits and contraindications
The surgeon’s decision-making process regarding choice of spinal rod material typically involves several factors, including intraoperative material bending properties, postoperative imaging capabilities, clinical experience, and training. Additional patient-specific considerations may include patient age, type, curve stiffness, and bone quality among other factors. ALTALYNE Ultra Rods address the unmet need with intraoperative rod flattening in pediatric spinal deformity surgeries in patients with AIS. Surgeons should be aware that increasing stiffness of the instrumentation in patients with osteoporosis is not recommended.
Do refer to the Instructions for Use (IFU) for more information including a complete list of indications and contraindications.
The rod strength and stiffness should be matched to the patient’s bone density in relation to adjacent level failure (PJF – PJK). The treatment of the osteoporotic spine with ALTALYNE rods is not recommended by the AO.
References
- US Preventive Services Task Force et al. Screening for adolescent idiopathic scoliosis: US Preventive Services Task Force Recommendation Statement. JAMA. 2018 Jan 9;319(2):165−172.
- Tatar O, Ekinci S, Akpancar S, et al. Current concepts of adolescent idiopathic scoliosis. Minerva Ortop Traumatol. 2016;67(3):156−162.
- Horne JP, Flannery R, Usman S. Adolescent idiopathic scoliosis: diagnosis and management. Am Fam Physician. 2014 Feb 1;89(3):193−198.
- Konieczny MR, Senyurt H, Krauspe R, et al. Epidemiology of adolescent idiopathic scoliosis. J Child Orthop. 2013 Feb 7 (1):3−9.
- Choudhry MN, Ahmad Z, Verma R. Adolescent idiopathic scoliosis. Open Orthop J. 2016 May 30;10:143−154.
- Sung S, Chae HW, Lee HS, et al. Incidence and surgery rate of idiopathic scoliosis: a Nationwide Database Study. Int J Environ Res Public Health. 2021 Aug 1;18(15):8152.
- Choudhry MN, Ahmad Z, Verma R. Adolescent idiopathic scoliosis. Open Orthop J. 2016 May 30;10:143−154.
- DePuy Synthes. EXPEDIUM® Advance Reduction Derotation System Vertebral Body Derotation Spondylolithesis Reduction Fracture Reduction Surgical Technique. 2018. Adaptiv No. 103407668 Rev. 1.
- DePuy Synthes. ALTALYNE Calculation of Rod Bending Yield Strength and Stiffness Memorandum, February 11, 2021. Adaptiv No. 103782175 Rev. 1.
- Yamada K, Sudo H, Iwasaki N, et al. Mechanical analysis of notch-free pre-bent rods for spinal deformity surgery. Spine (Phila Pa 1976). 2019 Mar 15;45(6):E312−E318.
- DePuy Synthes. Tolerance Analysis ALTALYNE Instruments. September 16, 2020. Adaptiv No. 103657526, Rev. 3
- DePuy Synthes. Mechanical Advantage Rod Cutter Memo. March 4, 2022. Adaptiv No. 103896297, Rev.1
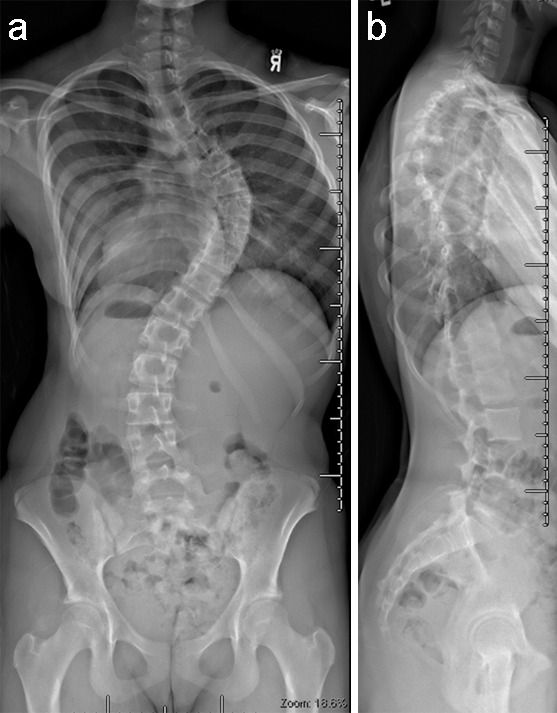
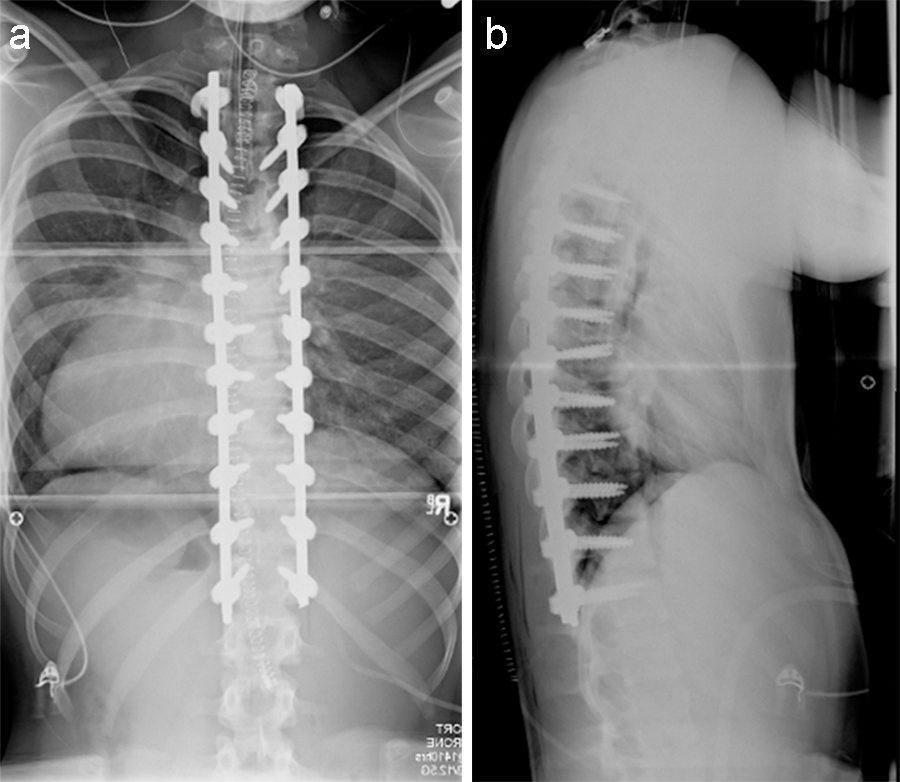
(Case provided by Firoz Miyanji, BC Children's Hospital, Vancouver, BC, Canada)
A 12-year-old girl with AIS underwent T2-T12 posterior spinal instrumentation and fusion.
Hazards and labeling
Due to varying countries’ legal and regulatory approval requirements, consult the appropriate local product labeling for approved intended use of the products described on this website. All devices on this website are approved by the AO Technical Commission. For logistical reasons, these devices may not be available in all countries worldwide at the date of publication.
Legal restrictions
This work was produced by AO Foundation, Switzerland. All rights reserved by AO Foundation. This publication, including all parts thereof, is legally protected by copyright.
Any use, exploitation or commercialization outside the narrow limits set forth by copyright legislation and the restrictions on use laid out below, without the publisher‘s consent, is illegal and liable to prosecution. This applies in particular to photostat reproduction, copying, scanning or duplication of any kind, translation, preparation of microfilms, electronic data processing, and storage such as making this publication available on Intranet or Internet.
Some of the products, names, instruments, treatments, logos, designs, etc referred to in this publication are also protected by patents, trademarks or by other intellectual property protection laws (eg, “AO” and the AO logo are subject to trademark applications/registrations) even though specific reference to this fact is not always made in the text. Therefore, the appearance of a name, instrument, etc without designation as proprietary is not to be construed as a representation by the publisher that it is in the public domain.
Restrictions on use: The rightful owner of an authorized copy of this work may use it for educational and research purposes only. Single images or illustrations may be copied for research or educational purposes only. The images or illustrations may not be altered in any way and need to carry the following statement of origin “Copyright by AO Foundation, Switzerland”.
Check www.aofoundation.org/disclaimer for more information.
If you have any comments or questions on the articles or the new devices, please do not hesitate to contact us.
“approved by AO Technical Commission” and “approved by AO”
The brands and labels “approved by AO Technical Commission” and “approved by AO”, particularly "AO" and the AO logo, are AO Foundation's intellectual property and subject to trademark applications and registrations, respectively. The use of these brands and labels is regulated by licensing agreements between AO Foundation and the producers of innovation products obliged to use such labels to declare the products as AO Technical Commission or AO Foundation approved solutions. Any unauthorized or inadequate use of these trademarks may be subject to legal action.
AO ITC Innovations Magazine
Find all issues of the AO ITC Innovations Magazine for download here.
Innovation Awards
Recognizing outstanding achievements in development and fostering excellence in surgical innovation.