DePuy Synthes Hammertoe Continuous Compression Implant
Christina Kabbash, Michael Swords
Hammertoe deformities are one of the most frequent deformities treated by foot and ankle surgeons. In terms of occurrence, pes planus foot posture has been associated with increased odds of developing such deformities [1]. Hammertoes are of concern in patients with diabetes, as the risk is increased for developing foot ulcers [2]. In rigid and structured hammertoe deformities not suited for nonoperative management, arthrodesis of the proximal interphalangeal (PIP) joint represents the standard treatment [3]. Temporary K-wire fixation is the most frequently used, low-cost fixation method for PIP joint fusion. However, reported complications associated with K-wires have prompted the development of new implants over the past decade. Most of these are intramedullary implants, such as cannulated screws, absorbable pins, or more sophisticated shape memory devices for continuous compression at the fusion site. To address weaknesses of intramedullary fixation concepts (eg, limited rotational stability), the Foot and Ankle Expert Group approved the first extramedullary continuous compression implant (CCI) to treat hammertoe deformities - the DePuy Synthes Hammertoe Continuous Compression Implant (DPS Hammertoe CCI).
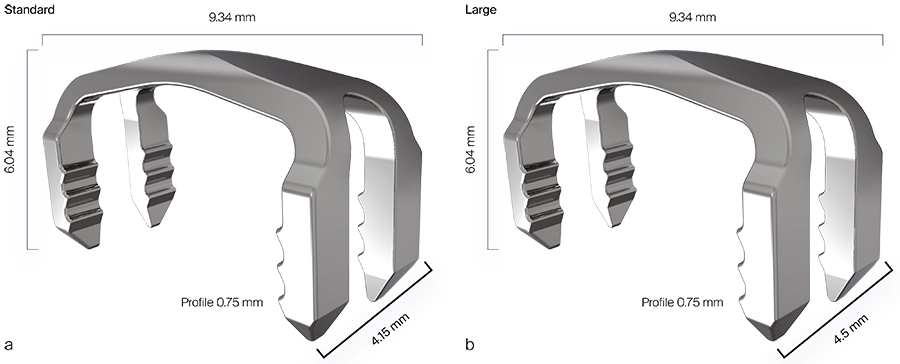
The DPS Hammertoe CCI has a staplelike design with four legs and a low profile connecting bridge. It is made of biocompatible Nitinol, a metal alloy of nickel and titanium, which is known for its superelastic properties and shape memory behavior. For more detailed information about the use of Nitinol implants and their function in orthopedic procedures see the corresponding article in the 2018 Innovations magazine.
The DPS Hammertoe CCI System is delivered to the operating room in a disposable, sterile kit containing the following components: Insertion Stick with pre-loaded implant (Fig 1), one Drilling Template, one Drill Pin, one K-wire, and three Locator Pins. The appropriate implant kit is chosen based on the diameter of the K-wire required for the toe.
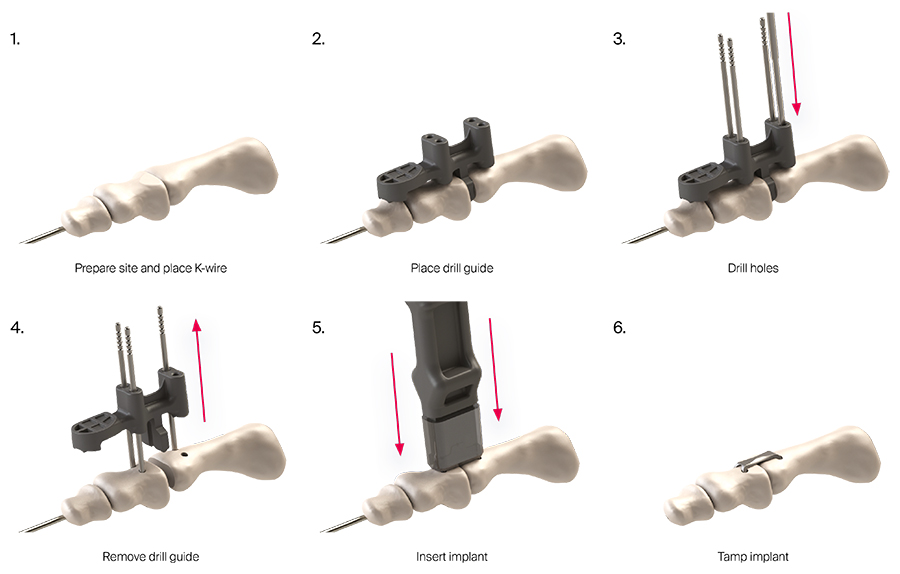
The straightforward surgical technique comprises six main steps to place the DPS Hammertoe CCI (Fig 2).
- Preparation of the fusion site and placement of the K-wire after proper phalange alignment.
- Placement of the Drilling Template dorsally over the PIP joint so that the spacer of the Drilling Template slides into the PIP joint and attaches securely onto the K-wire. Before any drilling is started the joint must be in the desired position.
- The Drill Pin with positive stop is used to drill the holes for the legs of the DPS Hammertoe CCI. Locator Pins are inserted into the first three drill holes after each drilling procedure to stabilize the Drilling Template and to keep the bone alignment.
- Removal of the Drilling Template leaving the Locator Pins in place to mark the positions of the drill holes and reduction of the PIP joint until the bones are flush. Proper rotational alignment can be established by verifying that the Locator Pins are parallel to each other.
- Insertion of the DPS Hammertoe CCI with the Insertion Stick after removal of the Locator Pins. The implant is automatically released as the legs of the implant are inserted into the holes and the implant is fully seated.
- The implant can be tamped further into the bone if needed.
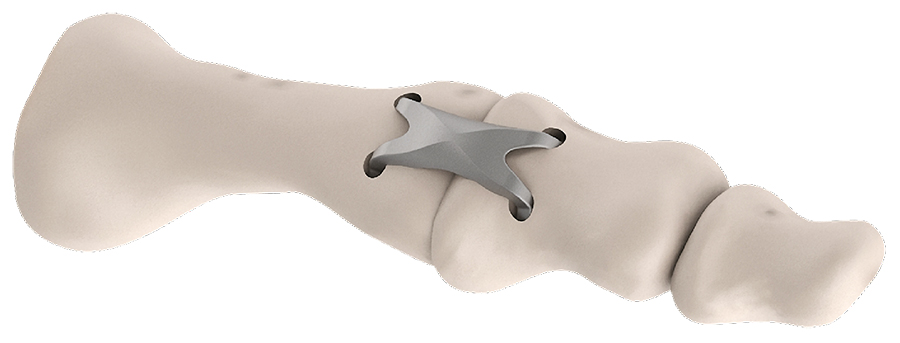
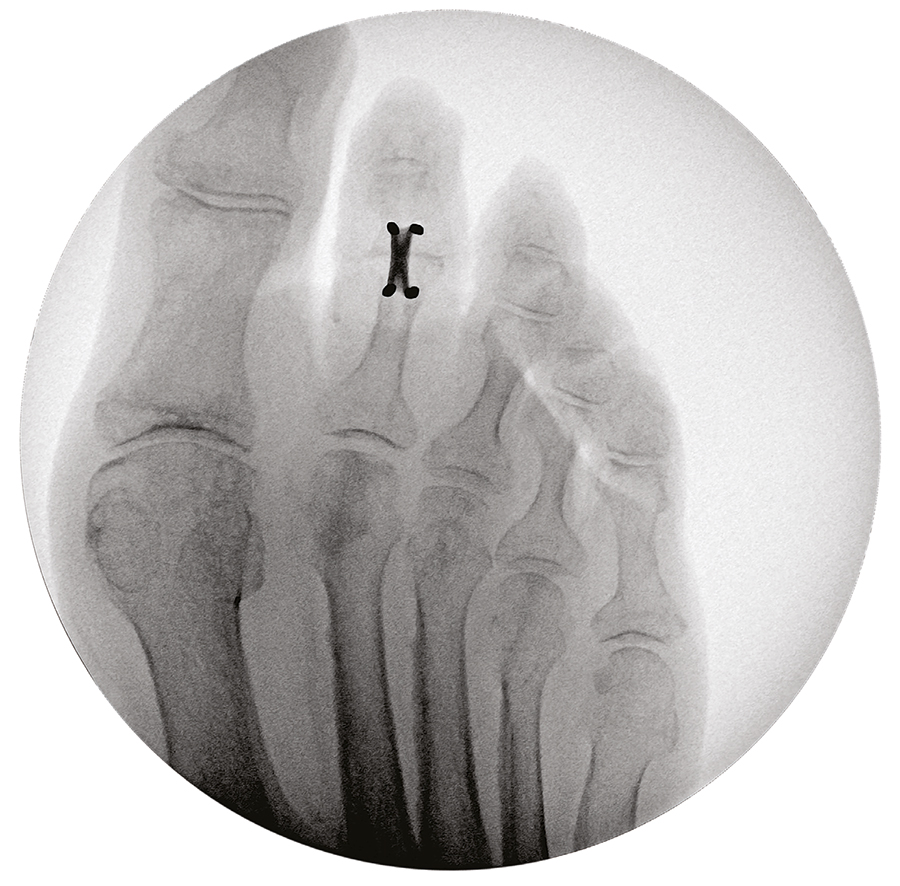
Once the DPS Hammertoe CCI is inserted into the bone and released from the Insertion Stick, the implant attempts to regain its original shape with converging legs thereby providing active continuous compression at the fusion site (Fig 3 and Fig 4). The implants do not require any external heating since they are already activated when preloaded on the insertion stick. The DPS Hammertoe CCI is not supposed to be reused after it has been discharged from the Insertion Stick because any processing, reprocessing, or mechanical manipulation may reduce the effectiveness of the implant. If for any reason the implant must be removed from the bone during the operation, then it must be replaced by a new one.
Due to the extramedullary position of the DPS Hammertoe CCI, bone stock is preserved, and the intramedullary pathway of callus formation is not affected. Furthermore, implant removal is simplified compared with intramedullary devices. Regarding patient aftertreatment, immobilization of the fusion site using routine methods (casting, splints, and so on) must be maintained until bone healing has occurred.
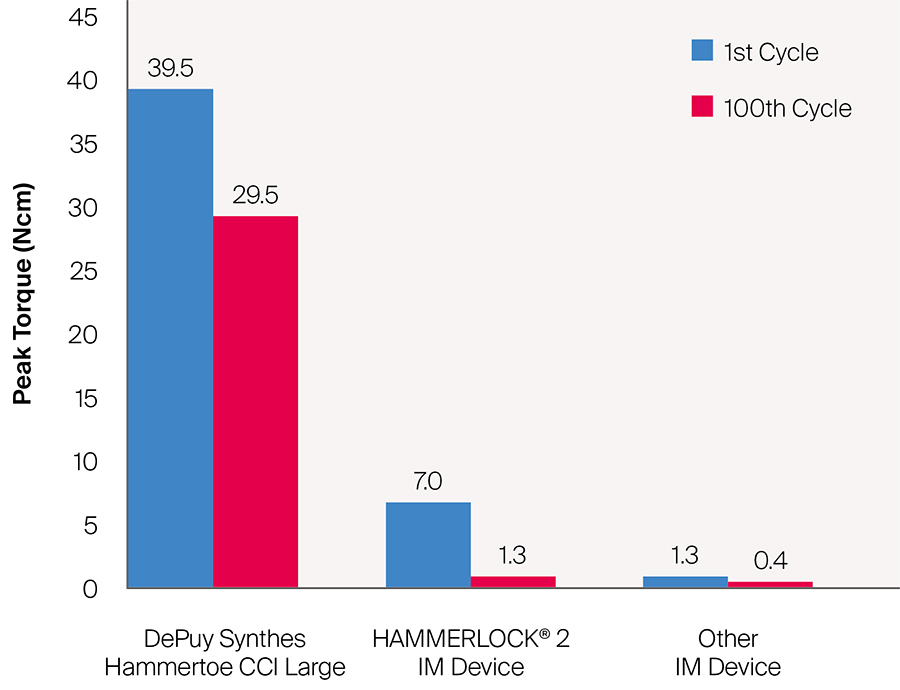
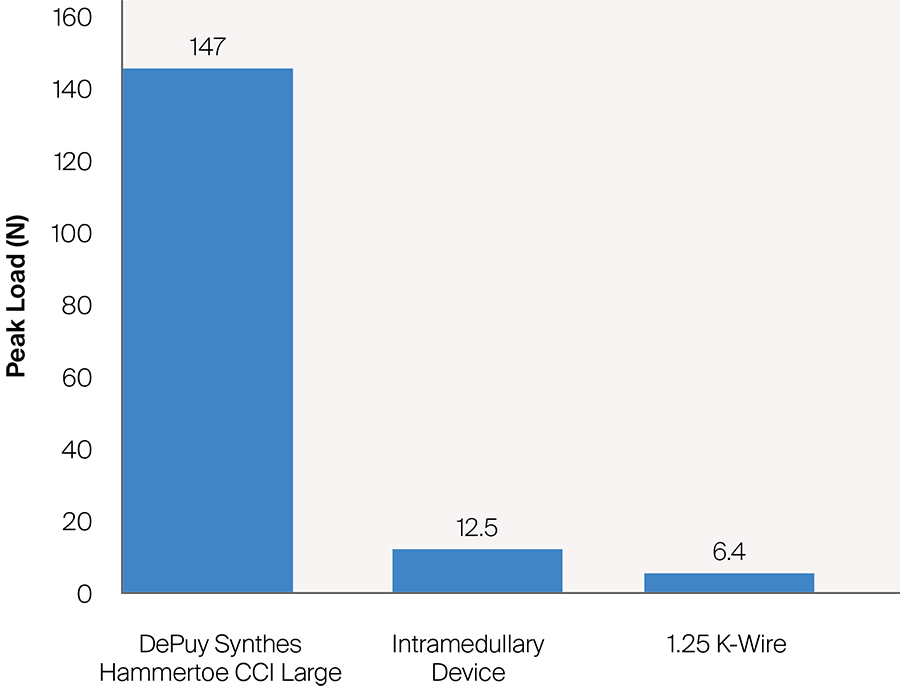
Since the DPS Hammertoe CCI is an extramedullary implant, it offers higher rotational stability than intramedullary implants (Fig 5). Its design with the four legs orthogonal to the bone axis and the active compression feature are beneficial for the distraction resistance (Fig 6).
The DPS Hammertoe CCI System is indicated for small bone reconstruction and fusion of the phalanges in toes. Contraindications include: comminuted bone surface that would militate against implant placement; pathologic conditions of bone such as osteopenia that would impair the ability to securely fix the implant; foreign body sensitivity to metals including nickel. Where material sensitivity is suspected, appropriate tests should be made prior to implantation.
In summary, the DPS Hammertoe CCI with its extramedullary application and continuous compression properties offers the potential to reduce the complication rate of PIP arthrodesis. However, the implant is more expensive than K-wires and in-depth cost-benefit studies are required to justify the use of the DPS Hammertoe CCI as standard treatment.
References
1. Hagedorn TJ, Dufour AB, Riskowski JL, et al. Foot disorders, foot posture, and foot function: The Framingham Foot Study. PLoS One. 2013 Sep;8(9):e74364.
2. Mavrogenis AF, Megaloikonomos PD, Antoniadou T, et al. Current concepts for the evaluation and management of diabetic foot ulcers. EFORT Open Rev. 2018;3(9):513-525.
3. Guelfi M, Pantalone A, Daniel JC, et al. Arthrodesis of proximal inter-phalangeal joint for hammertoe: intramedullary device options. J Orthop Traumatol. 2015 Dec;16(4):269-273.
Hazards and labeling
Due to varying countries’ legal and regulatory approval requirements, consult the appropriate local product labeling for approved intended use of the products described on this website. All devices on this website are approved by the AO Technical Commission. For logistical reasons, these devices may not be available in all countries worldwide at the date of publication.
Legal restrictions
This work was produced by AO Foundation, Switzerland. All rights reserved by AO Foundation. This publication, including all parts thereof, is legally protected by copyright.
Any use, exploitation or commercialization outside the narrow limits set forth by copyright legislation and the restrictions on use laid out below, without the publisher‘s consent, is illegal and liable to prosecution. This applies in particular to photostat reproduction, copying, scanning or duplication of any kind, translation, preparation of microfilms, electronic data processing, and storage such as making this publication available on Intranet or Internet.
Some of the products, names, instruments, treatments, logos, designs, etc referred to in this publication are also protected by patents, trademarks or by other intellectual property protection laws (eg, “AO” and the AO logo are subject to trademark applications/registrations) even though specific reference to this fact is not always made in the text. Therefore, the appearance of a name, instrument, etc without designation as proprietary is not to be construed as a representation by the publisher that it is in the public domain.
Restrictions on use: The rightful owner of an authorized copy of this work may use it for educational and research purposes only. Single images or illustrations may be copied for research or educational purposes only. The images or illustrations may not be altered in any way and need to carry the following statement of origin “Copyright by AO Foundation, Switzerland”.
Check www.aofoundation.org/disclaimer for more information.
If you have any comments or questions on the articles or the new devices, please do not hesitate to contact us.
“approved by AO Technical Commission” and “approved by AO”
The brands and labels “approved by AO Technical Commission” and “approved by AO”, particularly "AO" and the AO logo, are AO Foundation's intellectual property and subject to trademark applications and registrations, respectively. The use of these brands and labels is regulated by licensing agreements between AO Foundation and the producers of innovation products obliged to use such labels to declare the products as AO Technical Commission or AO Foundation approved solutions. Any unauthorized or inadequate use of these trademarks may be subject to legal action.
AO ITC Innovations Magazine
Find all issues of the AO ITC Innovations Magazine for download here.
Innovation Awards
Recognizing outstanding achievements in development and fostering excellence in surgical innovation.