VA LCP Two-Column Plate-Extra Long
Douglas Campbell, Thomas Fischer
VA LCP Two-Column Plate-Extra Long
In December 2016, AOTK Trauma approved the VA LCP Two-Column Extra Long Plate (Fig 1) designed for the treatment of intra- and extraarticular fractures, osteotomies, and nonunions and malunions of the distal radius. The plate is available with 7, 10, and 13 holes in the shaft and is indicated for fixation with or without extension into the radial diaphysis. Use of trial implants can assist with determining the plate size appropriate for the patient.
Cases 1 and 2 provided by Max Daniel Kauther and Marcel Dudda, Essen, Germany
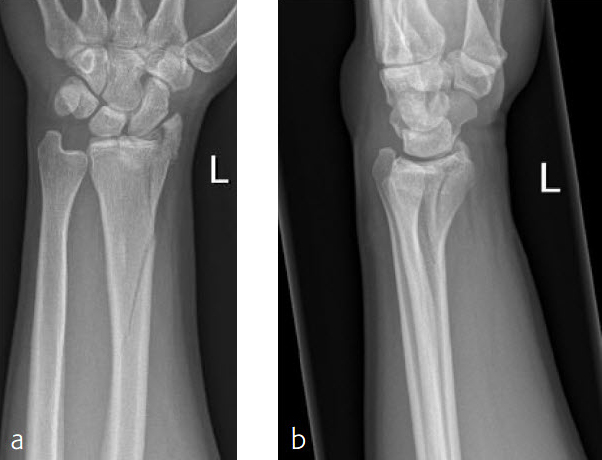
Case 1: Multifragmentary distal radius fracture with extension into the diaphysis
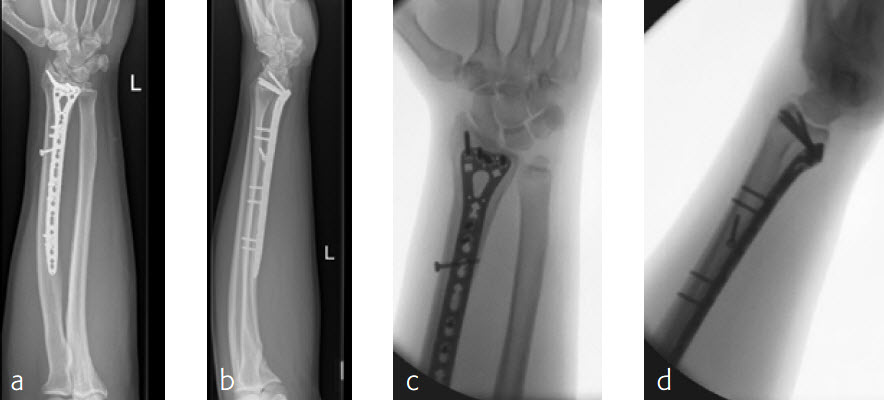
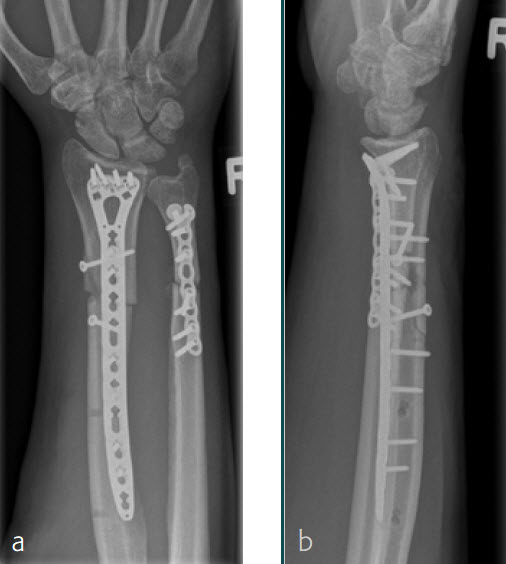
A 26-year-old man suffered a multifragmentary fracture of his left distal radius with extension into the diaphysis (AO23 C3.3) (Fig 2). The VA LCP Extra Long Two-Column plate was used for fixation (Fig 3). After initial immobilization, the plate provided a good postoperative fixation of the fracture.
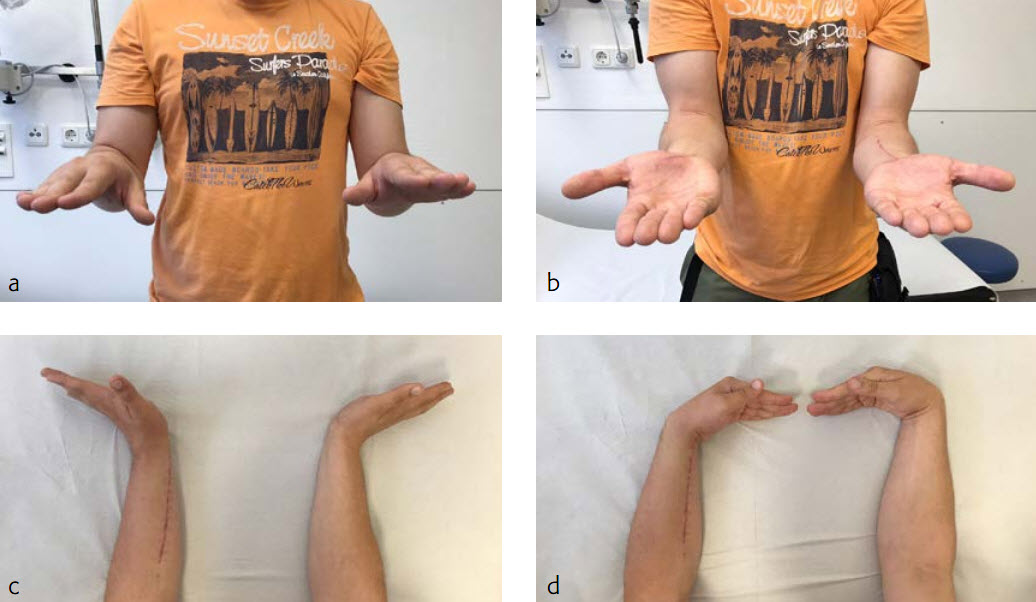
The fracture showed primary bone healing without callus formation. At the 3-month follow-up, the patient was full weight bearing with excellent clinical function (Fig 4). The radiological follow-up can be technically challenging due to the correct focus of the central ray.
Case 2: Open radius shaft fracture
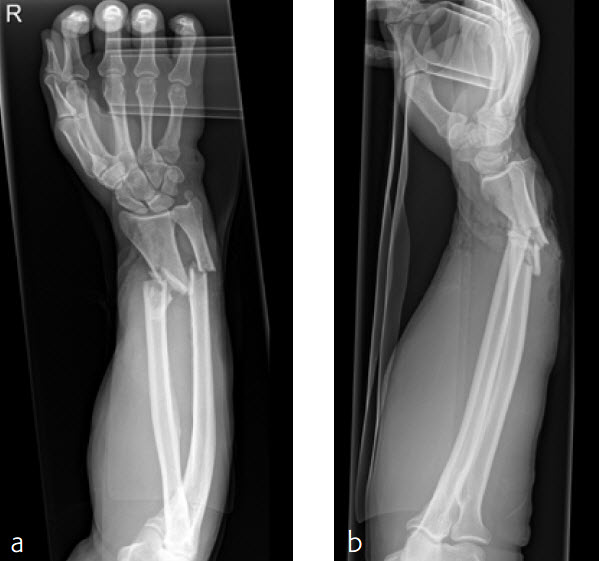
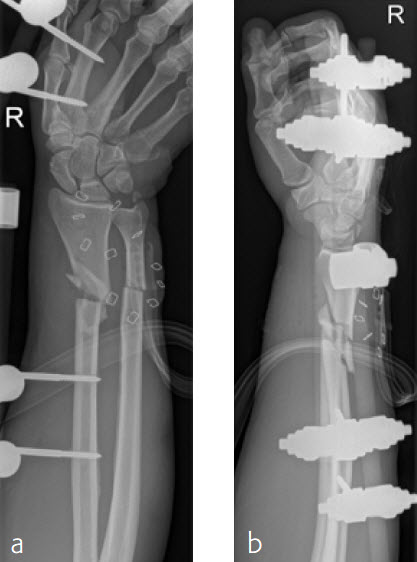
A 59-year-old farmer suffered a crush injury with an open forearm fracture (AO22 C2, Gustilo and Anderson IIIB) (Fig 5). Initial stabilization was carried out by external fixator (Fig 6). After four rounds of debridement and capillary ingrowth of a splitting skin graft at day 17, the VA LCP Extra Long Two-Column plate was used for fixation of the radius. A 2.7 mm LCP Condylar Plate was used for fixation of the ulna. The plates provided good stability for a functional after-treatment.
At the 3-month follow-up, the patient was full weight bearing with healing fractures (Fig 7).
Hazards and labeling
Due to varying countries’ legal and regulatory approval requirements, consult the appropriate local product labeling for approved intended use of the products described on this website. All devices on this website are approved by the AO Technical Commission. For logistical reasons, these devices may not be available in all countries worldwide at the date of publication.
Legal restrictions
This work was produced by AO Foundation, Switzerland. All rights reserved by AO Foundation. This publication, including all parts thereof, is legally protected by copyright.
Any use, exploitation or commercialization outside the narrow limits set forth by copyright legislation and the restrictions on use laid out below, without the publisher‘s consent, is illegal and liable to prosecution. This applies in particular to photostat reproduction, copying, scanning or duplication of any kind, translation, preparation of microfilms, electronic data processing, and storage such as making this publication available on Intranet or Internet.
Some of the products, names, instruments, treatments, logos, designs, etc referred to in this publication are also protected by patents, trademarks or by other intellectual property protection laws (eg, “AO” and the AO logo are subject to trademark applications/registrations) even though specific reference to this fact is not always made in the text. Therefore, the appearance of a name, instrument, etc without designation as proprietary is not to be construed as a representation by the publisher that it is in the public domain.
Restrictions on use: The rightful owner of an authorized copy of this work may use it for educational and research purposes only. Single images or illustrations may be copied for research or educational purposes only. The images or illustrations may not be altered in any way and need to carry the following statement of origin “Copyright by AO Foundation, Switzerland”.
Check www.aofoundation.org/disclaimer for more information.
If you have any comments or questions on the articles or the new devices, please do not hesitate to contact us.
“approved by AO Technical Commission” and “approved by AO”
The brands and labels “approved by AO Technical Commission” and “approved by AO”, particularly "AO" and the AO logo, are AO Foundation's intellectual property and subject to trademark applications and registrations, respectively. The use of these brands and labels is regulated by licensing agreements between AO Foundation and the producers of innovation products obliged to use such labels to declare the products as AO Technical Commission or AO Foundation approved solutions. Any unauthorized or inadequate use of these trademarks may be subject to legal action.
AO ITC Innovations Magazine
Find all issues of the AO ITC Innovations Magazine for download here.
Innovation Awards
Recognizing outstanding achievements in development and fostering excellence in surgical innovation.