Stand-alone anterior interbody fusion with the Synfix Evolution
Paul Heini
The Synfix Evolution secured spacer system (Fig 1,2) is a stand-alone anterior interbody fusion (ALIF) implant that is intended for use in patients with degenerative disc disease (DDD). It employs the Synfix implant technology, which has been used clinically in the Synfix LR implant since 2004. This technology is a zero-profile construct that includes four diverging locking screws. This design negates, in most circumstances, the need for additional fixation.
Implant overview
The Synfix Evolution implant has been designed to preserve the biomechanical stability of the Synfix LR implant by delivering the following:
- An integrated titanium plate with four diverging locking screws that form a fixed angle construct, creating a wedge of bone designed as an anchor to potentially prevent fixation failure
- A nonrigid connection between locking plate and PEEK spacer allowing for load sharing
- PEEK spacer with elastic modulus similar to cortical bone
- Self-tapping cortical threads
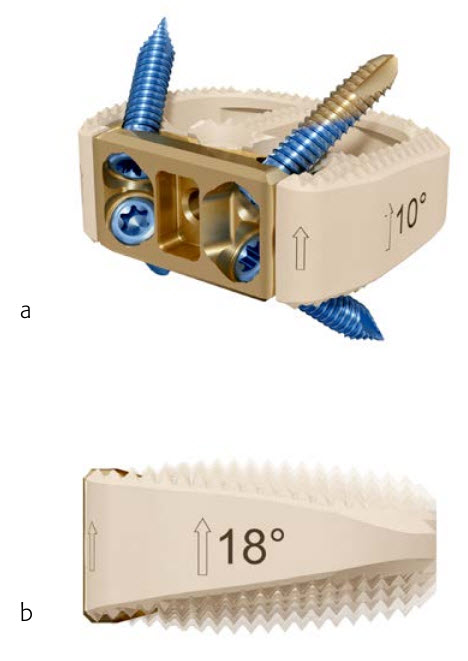
Fig 1 Synfix Evolution implant. Frontal view showing the zero-profile integrated titanium plate, fine and blunt tip self-tapping screws, and a graft retention ridge to enhance graft retention (a). The four lordotic angulations of 6, 10, 14, and 18 support sagittal alignment restoration (b).
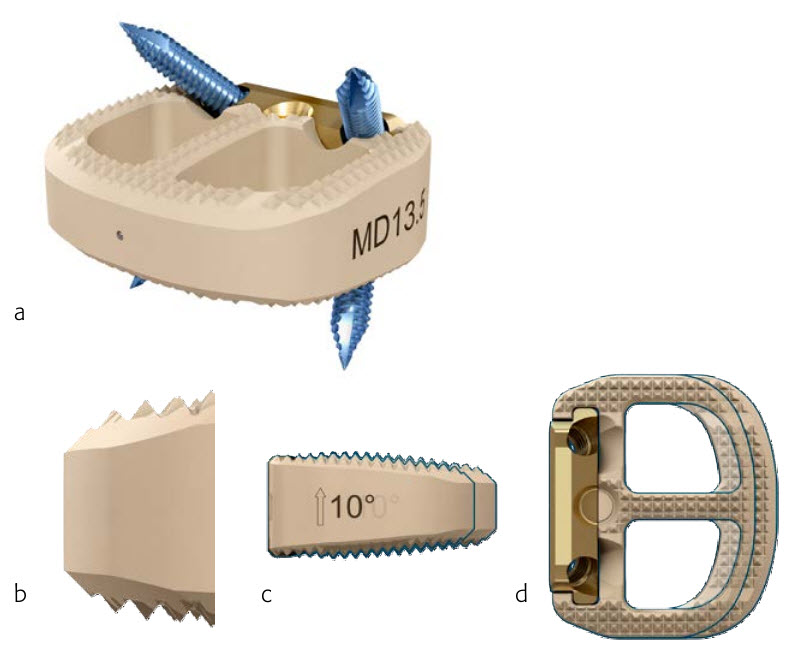
Fig 2 Synfix Evolution implant posterior view (a). The implant uses a PEEK spacer to facilitate radiographic assessment of fusion, and comes in a wide range of implant heights. The bullet nose allows for ease of insertion (b). The deeper footprint option provides 3 mm of additional depth in the AP direction to accommodate varied anatomies (c-d).
Comprehensive implant portfolio
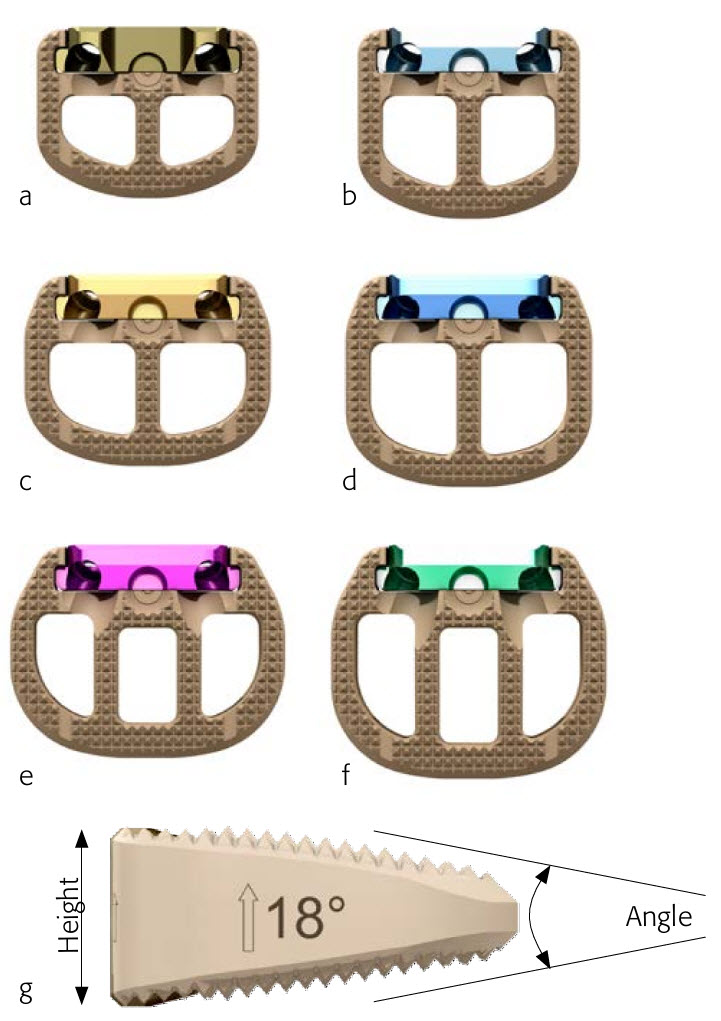
Fig 3: The Synfix implant portfolio includes:
- 126 implants to support an optimal fit and fill of disc space and restoration of sagittal alignment (Fig 3)
- 6 footprints
- 6 heights
- 4 angles
Instrumentation overview
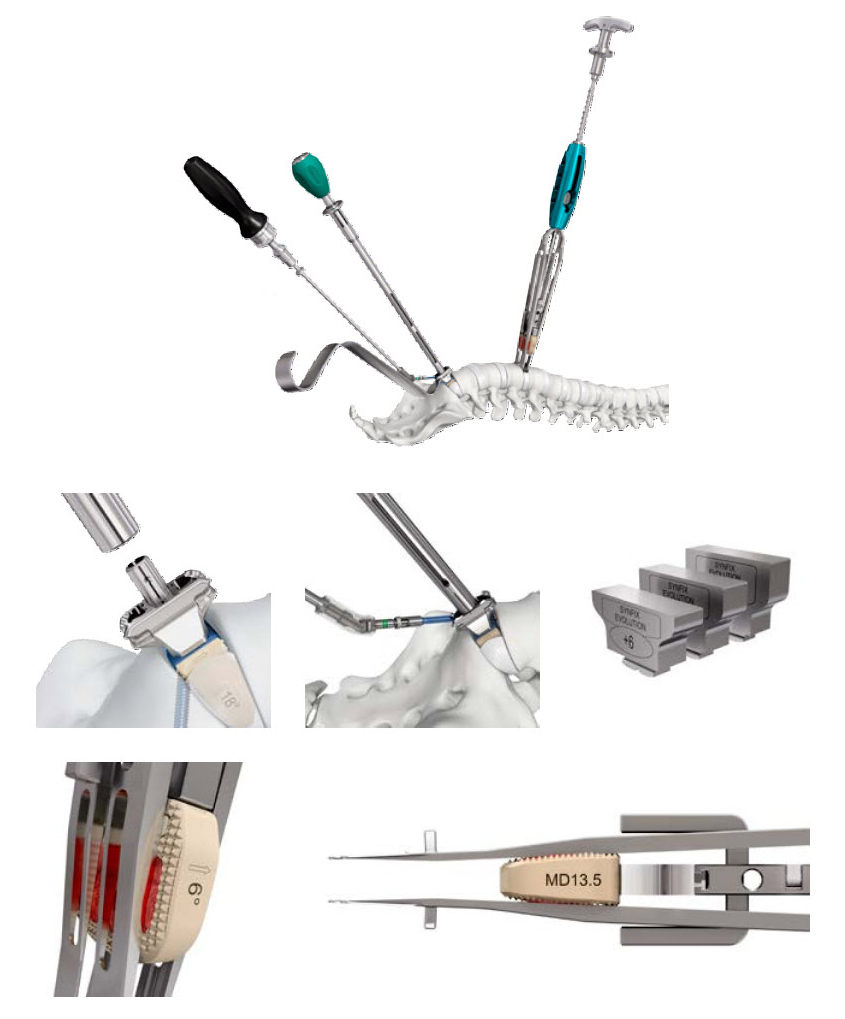
The Synfix Evolution instruments were conceived and designed to simplify and minimize the number of instrumentation steps, while maintaining a high level of safety and precision (Fig 4).
Case provided by Paul Heini, Bern, Switzerland
Case: 47-year-old patient with chronic back pain
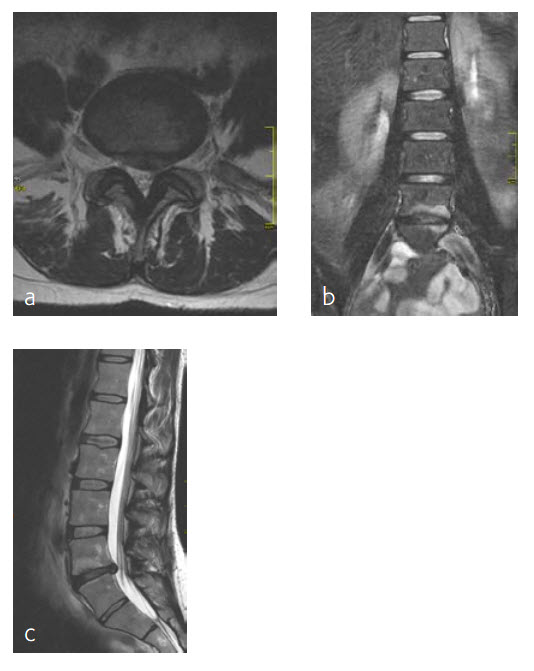
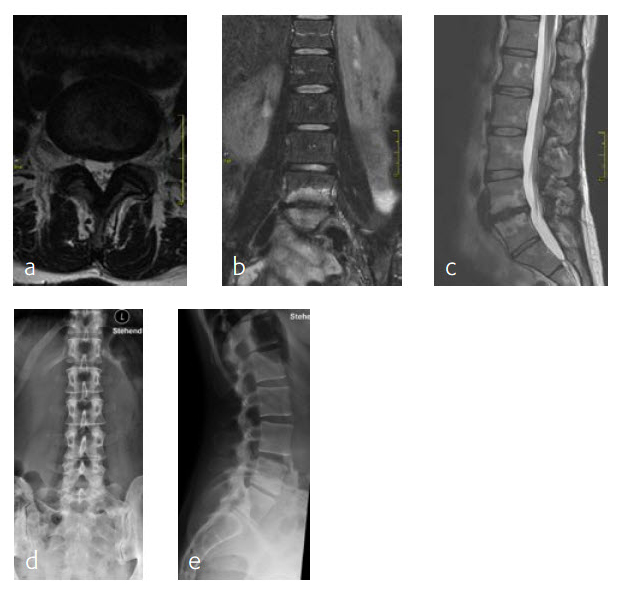
A 47-year-old woman was suffering chronic low back pain for several years, with severe pain attacks and with uncontrolled movements becoming increasingly disabling due to DD and erosive osteochondrosis at L4/L5. Condition after sciatica due to a disc herniation L4/L5, having nonoperative treatment. The MRI findings progressive compared to April 2014 are shown (Fig 5 and 6).
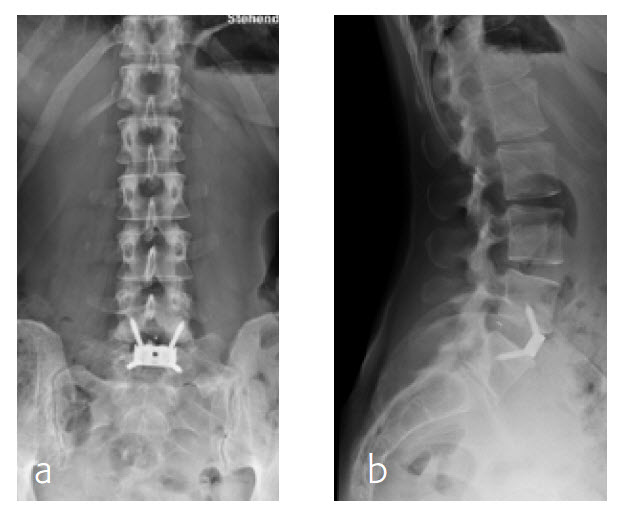
Anterior lumbar interbody fusion surgery was undertaken in July 2016 using the Synfix Evolution with InductOs (6 mg) (Fig 7). Intraoperative: routine operation, no complications. Postoperative: uneventful postoperative course.
6-month follow-up
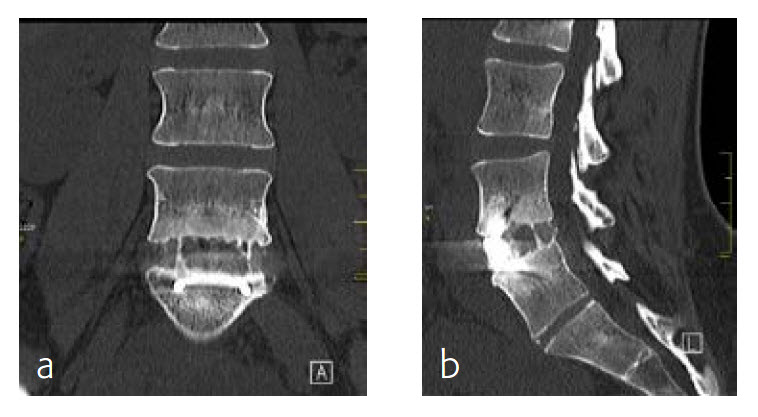
By the planned 6-month follow-up, the patient was pain free and fully active. Evaluation with CT scans was undertaken, consolidation was starting (Fig 8).
Hazards and labeling
Due to varying countries’ legal and regulatory approval requirements, consult the appropriate local product labeling for approved intended use of the products described on this website. All devices on this website are approved by the AO Technical Commission. For logistical reasons, these devices may not be available in all countries worldwide at the date of publication.
Legal restrictions
This work was produced by AO Foundation, Switzerland. All rights reserved by AO Foundation. This publication, including all parts thereof, is legally protected by copyright.
Any use, exploitation or commercialization outside the narrow limits set forth by copyright legislation and the restrictions on use laid out below, without the publisher‘s consent, is illegal and liable to prosecution. This applies in particular to photostat reproduction, copying, scanning or duplication of any kind, translation, preparation of microfilms, electronic data processing, and storage such as making this publication available on Intranet or Internet.
Some of the products, names, instruments, treatments, logos, designs, etc referred to in this publication are also protected by patents, trademarks or by other intellectual property protection laws (eg, “AO” and the AO logo are subject to trademark applications/registrations) even though specific reference to this fact is not always made in the text. Therefore, the appearance of a name, instrument, etc without designation as proprietary is not to be construed as a representation by the publisher that it is in the public domain.
Restrictions on use: The rightful owner of an authorized copy of this work may use it for educational and research purposes only. Single images or illustrations may be copied for research or educational purposes only. The images or illustrations may not be altered in any way and need to carry the following statement of origin “Copyright by AO Foundation, Switzerland”.
Check www.aofoundation.org/disclaimer for more information.
If you have any comments or questions on the articles or the new devices, please do not hesitate to contact us.
“approved by AO Technical Commission” and “approved by AO”
The brands and labels “approved by AO Technical Commission” and “approved by AO”, particularly "AO" and the AO logo, are AO Foundation's intellectual property and subject to trademark applications and registrations, respectively. The use of these brands and labels is regulated by licensing agreements between AO Foundation and the producers of innovation products obliged to use such labels to declare the products as AO Technical Commission or AO Foundation approved solutions. Any unauthorized or inadequate use of these trademarks may be subject to legal action.
AO ITC Innovations Magazine
Find all issues of the AO ITC Innovations Magazine for download here.
Innovation Awards
Recognizing outstanding achievements in development and fostering excellence in surgical innovation.