Colibri II - Power For Spine
Philip Horsting, Jean Ouellet
The Expedium 5.5 Power Instrument Set is designed to augment the current instrument set for the Expedium 5.5 implant family of products in three main areas:
- Enhanced surgeon ergonomics and health
- Speed and efficiency
- Safety and accuracy
Enhanced surgeon ergonomics and health
It is a well-known fact that spinal deformity surgeries are long procedures with repetitive forceful maneuvers often in a less than ideal ergonomic position. Such repetitive tasks lead to chronic overuse injury. Auerbach [1] quantified the type of work-related injuries that spine surgeons experienced over their careers (Table 1).
The conclusion that can be derived from research such as in Table 1 is that compared with disease estimates in the general population, spine surgeons have a higher prevalence of MSDs.
The instrument length in the Expedium 5.5 Power Instrument Set is designed to improve surgeon ergonomics and provides a more comfortable shoulder and torso positioning during surgical procedures. Additionally, the powered hand piece reduces the number of repetitive motions that can lead to surgeon fatigue or overuse injuries.
Improving ergonomics as well as decreasing the repetitive motions of the upper extremity would indeed improve the health of operating clinicians.
There is a growing need for surgeons to adapt their practice to incorporate better ergonomics. This can be achieved with good training programs and attaining a good understanding of the risks and benefits of using power in spine surgery.
Speed and efficiency
The application of powered instruments also offers potential timesaving for each screw implant. The greatest cumulative benefit is expected during deformity procedures.
Safety and accuracy
The power hand piece is designed to facilitate steadier hand positioning during pedicle preparation and screw delivery, potentially reducing the off-axis motion associated with manual pedicle preparation and screw delivery.
Seehausen [2] showed that the use of power tools to create pedicle tracts and place pedicle screws was associated with shorter fluoroscopy times and a lower revision rate compared with using manual tools. He showed that both techniques posed similar low risks of injury to the patient.
Power drilling also guarantees high accuracy of the navigation system
as low downward forces are applied to the spine.
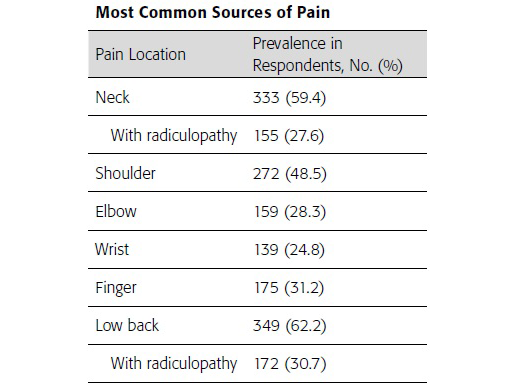
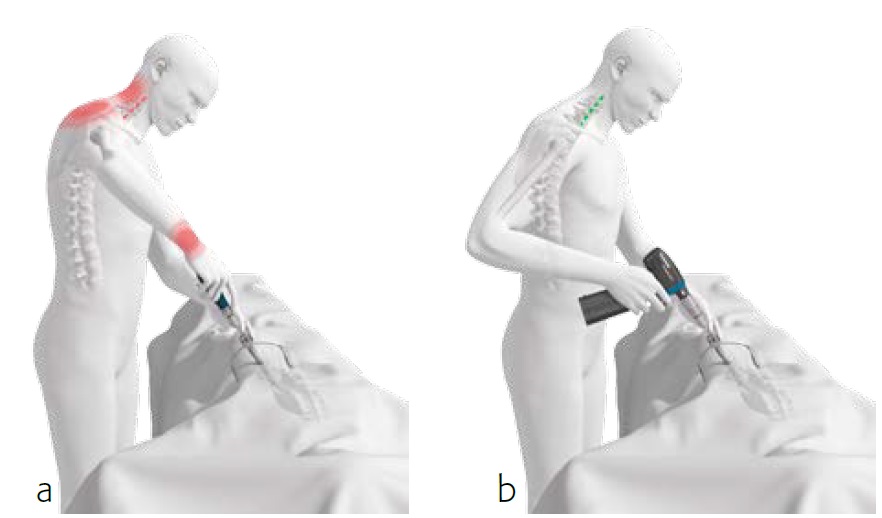
Comparison of surgeon posture using manual and powered instruments
During pedicle preparation and screw delivery, powered instrumentation allows the surgeon to maintain a low shoulder position, an elbow position close to the torso, and decreases the amount of lateral bending often seen when using manual instrumentation (Figs 1 and 2). Maintaining alignment to the torso while minimizing the extension of the
upper extremities provides an opportunity to reduce muscle recruitment and surgeon fatigue. Similarly, using powered instruments reduces the number of repetitive motions required during spine surgery, reducing energy expenditure and decreasing the potential for musculoskeletal disorders associated with overuse [1].
References
- Auerbach JD , Weidner ZD, Milby AH, et al. Musculoskeletal disorders among spine surgeons-results of a survey of the Scoliosis Research Society Membership. Spine. 2011 Dec; 36(26):e17151721.
- Seehausen DA, Skaggs DL, Andras LM, et al. Safety and efficacy of powerassistedpedicle tract preparation and screw placement. Spine Deformity. 2015 March;3(2):159165.
Expedium 5.5 spine system
The Expedium 5.5 Power Instrument Set will address the key ergonomic and repetitive use injuries for surgeons performing high volumes of deformity or degenerative surgery as well as potentially reducing OR time and improving accuracy. This is accomplished through the ability to utilize the Expedium 5.5 product portfolio (ie, polyaxial, monoaxial, uniplanar screws) with powered pedicle preparation and screw delivery.
The instruments were developed in close collaboration with deformity surgeons. Through optimization of instrument length and geometry, shoulder, elbow, and trunk positioning is improved when compared to manual instrumentation.
Product overview
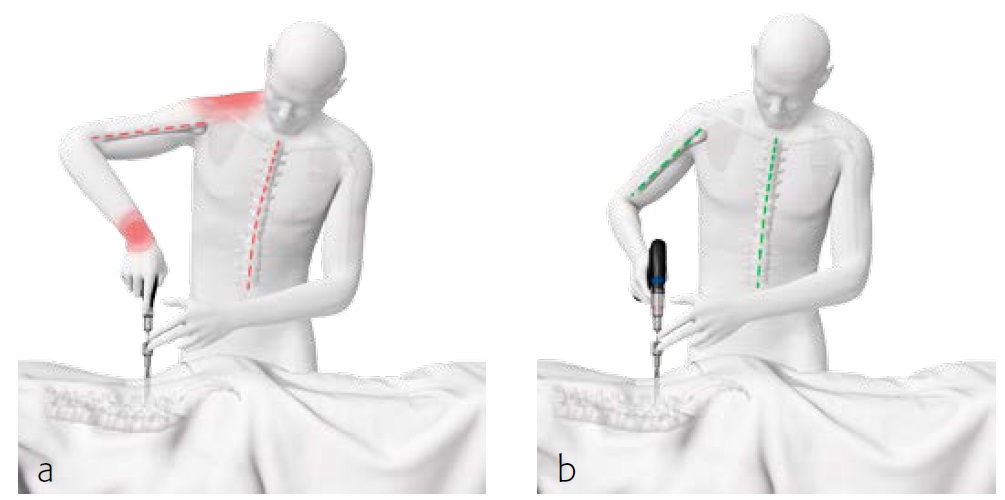
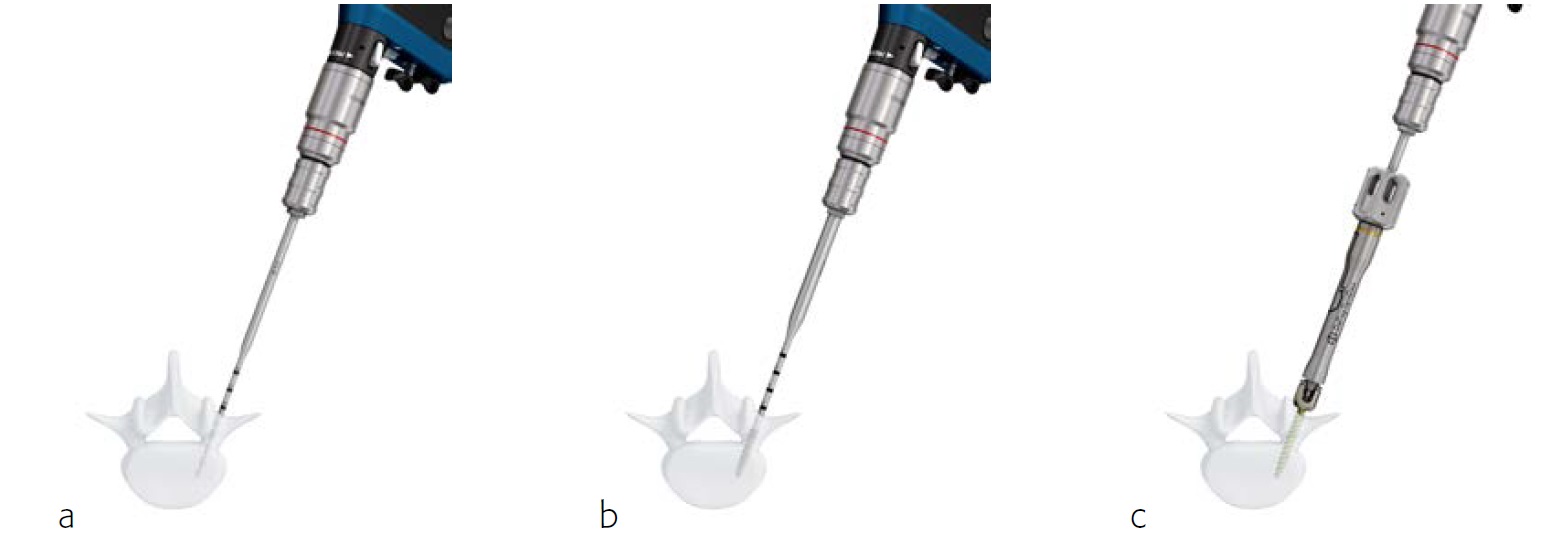
The Expedium 5.5 power system uses the Colibri II power device (Fig 3), (which is known as the Small Battery Drive II (SBDII) in the USA). The features and benefits of using the system are shown.
The powered instruments for the Expedium 5.5 system enable powered drilling, tapping, and screw delivery with a cordless hand piece, quick coupling attachment, and optimized instrumentation (Fig 4).
Case provided by Jean Ouellet, Montreal, Canada
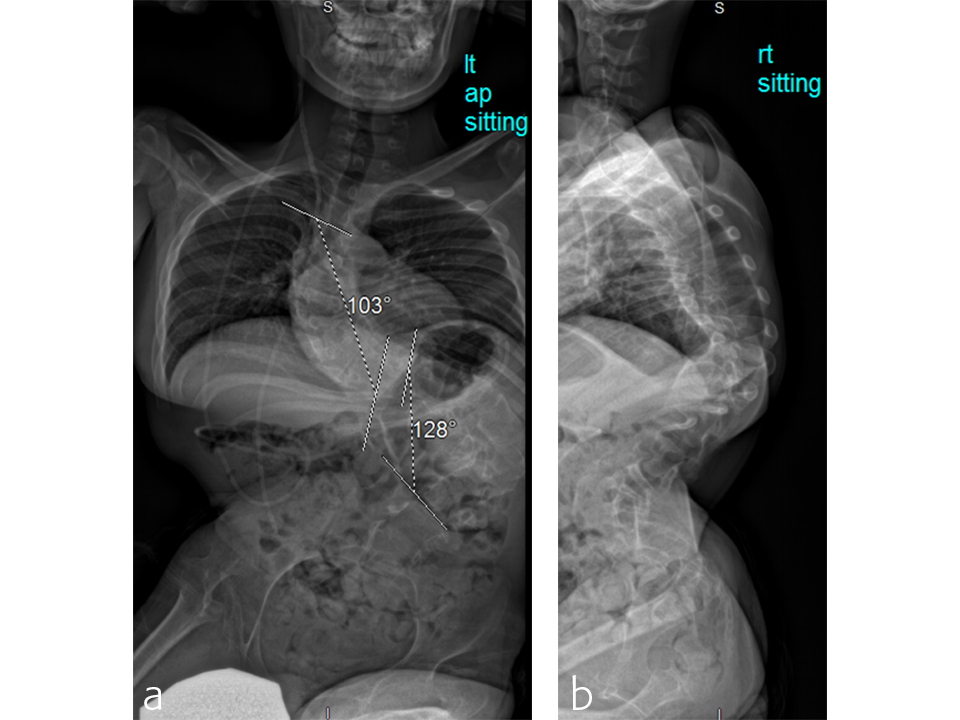
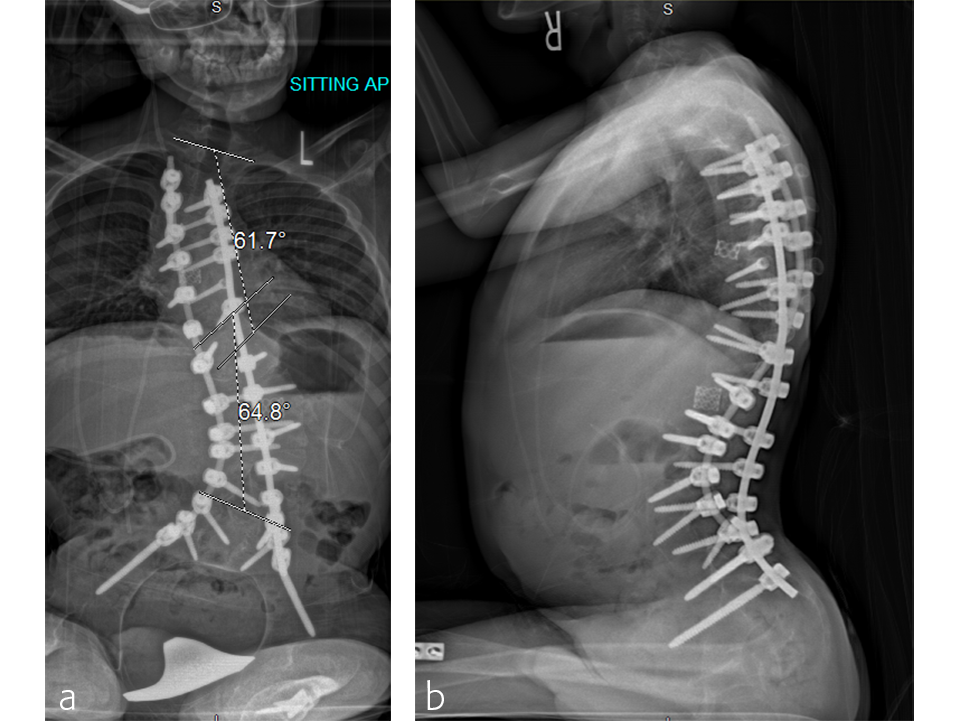
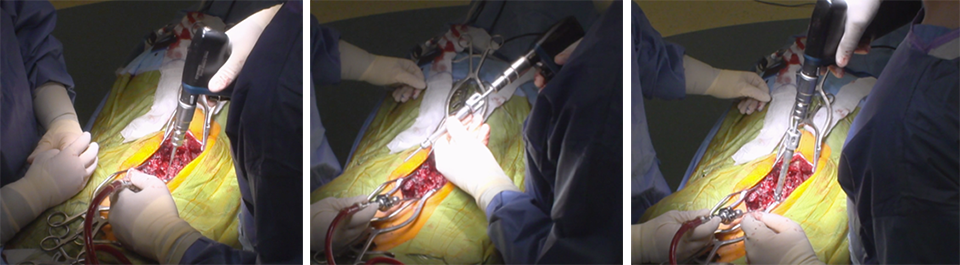
Case: A two-level VCR
A T2 to pelvis, two-level vertebral column resection (VCR) was completed at the McGill University Health Center in 7 hours (Figs 5–7).
Important Considerations for the Safe & Efficient Use of Powered Pedicle Screw Insertion
Hazards and labeling
Due to varying countries’ legal and regulatory approval requirements, consult the appropriate local product labeling for approved intended use of the products described on this website. All devices on this website are approved by the AO Technical Commission. For logistical reasons, these devices may not be available in all countries worldwide at the date of publication.
Legal restrictions
This work was produced by AO Foundation, Switzerland. All rights reserved by AO Foundation. This publication, including all parts thereof, is legally protected by copyright.
Any use, exploitation or commercialization outside the narrow limits set forth by copyright legislation and the restrictions on use laid out below, without the publisher‘s consent, is illegal and liable to prosecution. This applies in particular to photostat reproduction, copying, scanning or duplication of any kind, translation, preparation of microfilms, electronic data processing, and storage such as making this publication available on Intranet or Internet.
Some of the products, names, instruments, treatments, logos, designs, etc referred to in this publication are also protected by patents, trademarks or by other intellectual property protection laws (eg, “AO” and the AO logo are subject to trademark applications/registrations) even though specific reference to this fact is not always made in the text. Therefore, the appearance of a name, instrument, etc without designation as proprietary is not to be construed as a representation by the publisher that it is in the public domain.
Restrictions on use: The rightful owner of an authorized copy of this work may use it for educational and research purposes only. Single images or illustrations may be copied for research or educational purposes only. The images or illustrations may not be altered in any way and need to carry the following statement of origin “Copyright by AO Foundation, Switzerland”.
Check www.aofoundation.org/disclaimer for more information.
If you have any comments or questions on the articles or the new devices, please do not hesitate to contact us.
“approved by AO Technical Commission” and “approved by AO”
The brands and labels “approved by AO Technical Commission” and “approved by AO”, particularly "AO" and the AO logo, are AO Foundation's intellectual property and subject to trademark applications and registrations, respectively. The use of these brands and labels is regulated by licensing agreements between AO Foundation and the producers of innovation products obliged to use such labels to declare the products as AO Technical Commission or AO Foundation approved solutions. Any unauthorized or inadequate use of these trademarks may be subject to legal action.
AO ITC Innovations Magazine
Find all issues of the AO ITC Innovations Magazine for download here.
Innovation Awards
Recognizing outstanding achievements in development and fostering excellence in surgical innovation.