Expedium Verse System
Philip Horsting, Jean Ouellet
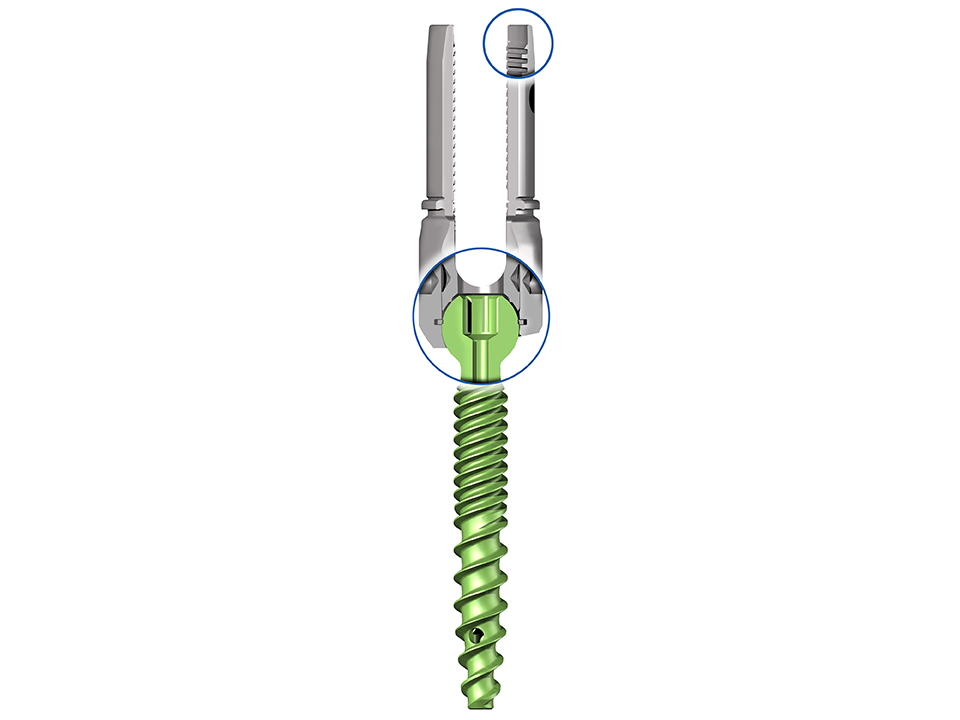
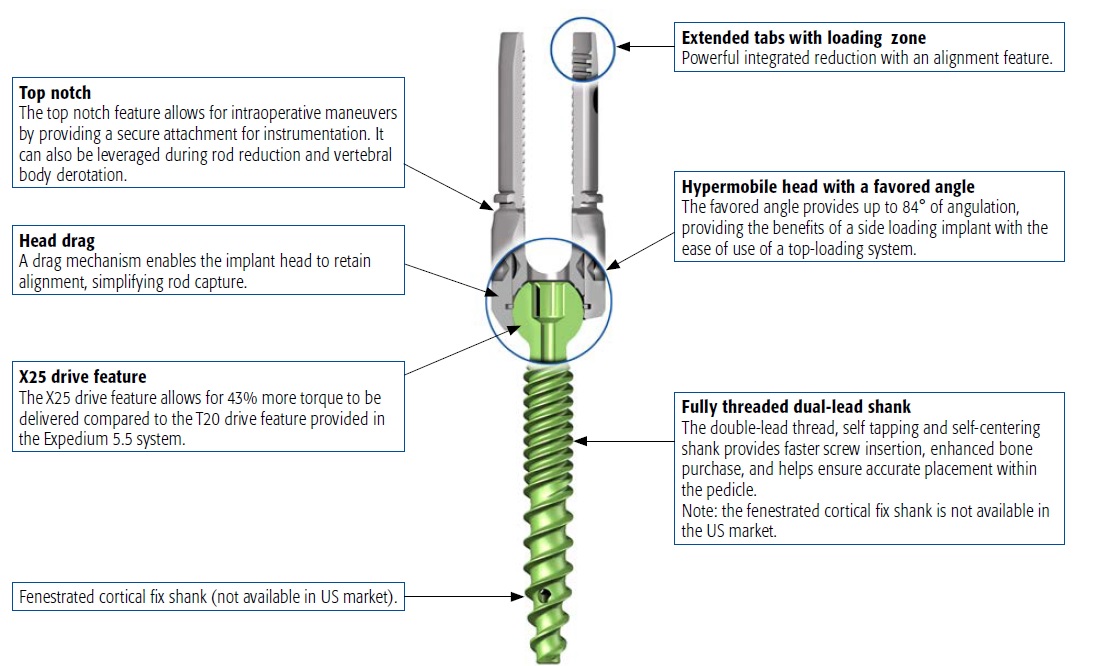
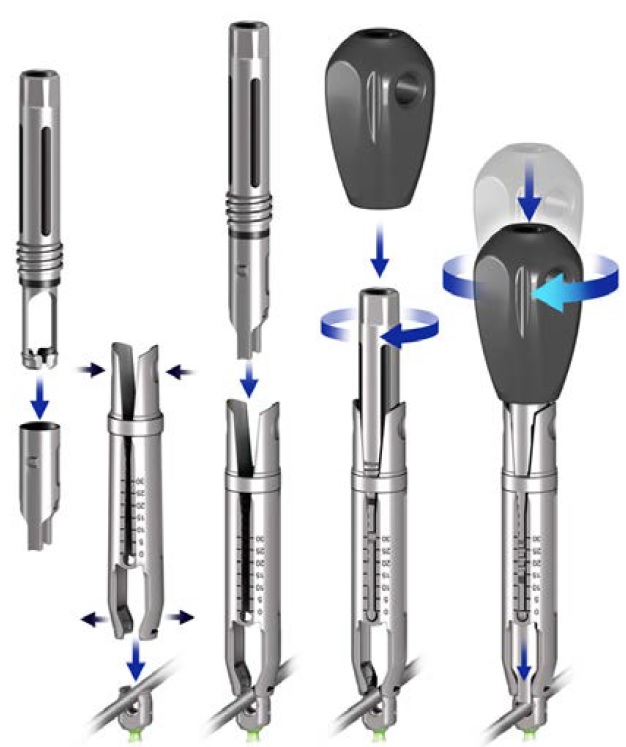
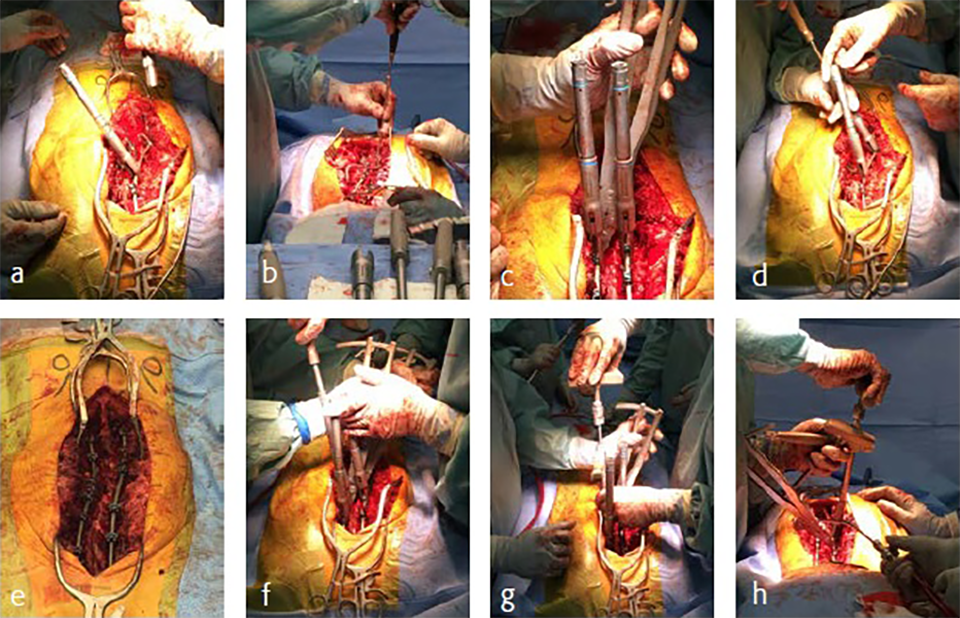
The Expedium Verse pedicle screw system (Fig 1 below) combines the attributes of multiple screw types (side-loading, monoaxial, polyaxial, uniplanar, and reduction screws) while offering intraoperative flexibility allowing surgical staff to address unplanned circumstances with one versatile implant. This ultimately results in the delivery of a more predictable intraoperative experience for the treatment of both adult and paediatric spine deformity.
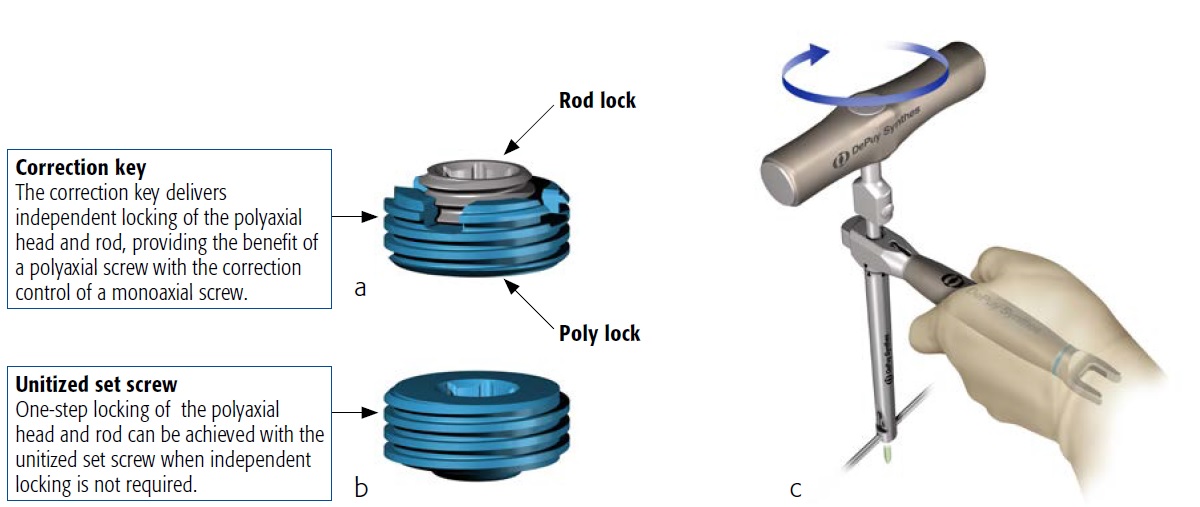
The Expedium Verse implant incorporates technology that allows for converting the polyaxial screw into a monoaxial screw while allowing for translation along the rod. The correction key is used as a locking mechanism that provides independent locking of polyaxial head and rod.
Easier rod capture with powerful and controlled correction
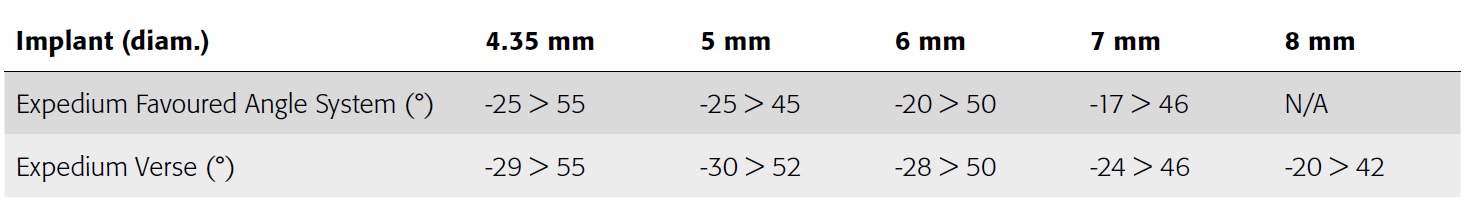
The "hypermobility" or increased angulation (Table 1) of the polyaxial head in combination with the reduction tabs simplify rod capture while providing a powerful threaded reduction mechanism that accommodates controlled approximation of the spine to the rod. The pedicle screws serve as a powerful instrument in the facilitation of correction maneuvers.
The result is a great reduction in the number of instruments required for fusion procedures, potentially simplifying the surgical instrument table and reducing the costs associated with the sterilization process. The Expedium Verse pedicle screw can be converted into a monoaxial implant while allowing the screw to articulate around the rod. Tighten the poly lock of the correction key with the torque limiting handle while applying counter torque to lock the polyaxial head (Fig 2).
Instrument design and set configuration
Through the redesign and feature enhancement on both the instruments and implants, it was possible to significantly reduce the number of instruments when compared to a traditional system such as the Expedium 5.5, enabling a shift away from instrument based correction methods to a more implant based procedure.
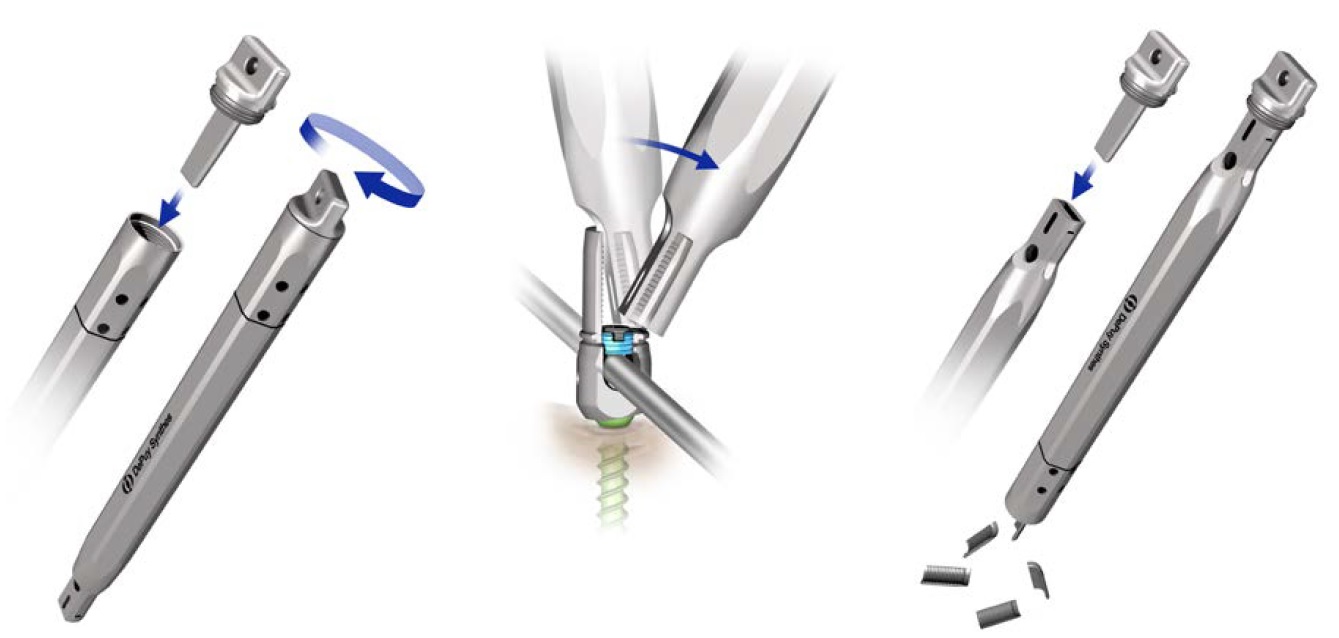
The polyaxial screwdriver modular design also allows for intraoperative assembly and includes tissue protection sleeves (Fig 3).
The Expedium Verse system provides a flex clip reducer (Fig 4) known from the Expedium 5.5 system for surgeons in case a reduction tab is accidentally or intentionally removed prior to reducing the rod into the screw head.
A tab remover has been provided for removal of the Expedium Verse screw reduction tabs at the completion of the procedure (Fig 5).
Please refer to Expedium Verse IFU for complete listing of warnings, contraindications and precautions.
Cases provided by Philip Horsting, Nijmegen, Netherlands
Case 1: Teenage boy with intellectual disability
Twelve months prior to his first visit to our clinic, the father of this 17-year-old intellectually disabled boy found a scoliosis, later confirmed by his therapist.
The patient was physically grown comparable to his age but mentally functioned at a 2-year-old level. No syndromic diagnosis was made after visits to a pediatrician. He had been diagnosed with severe autism. Behavioural changes might be suggestive of pain. The patient was unable to specifically indicate pain or (progressive) limitations.
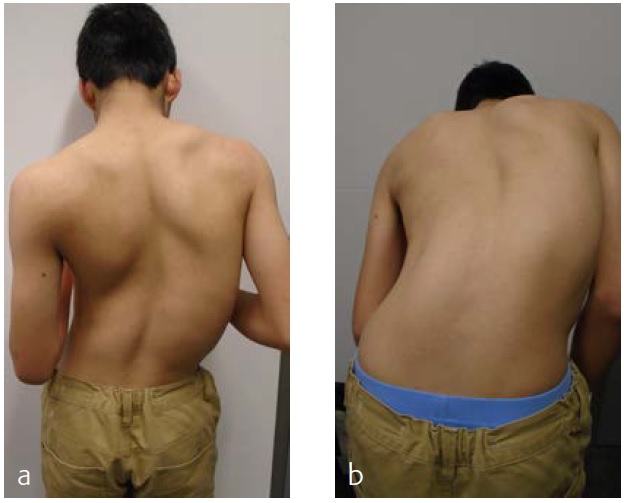
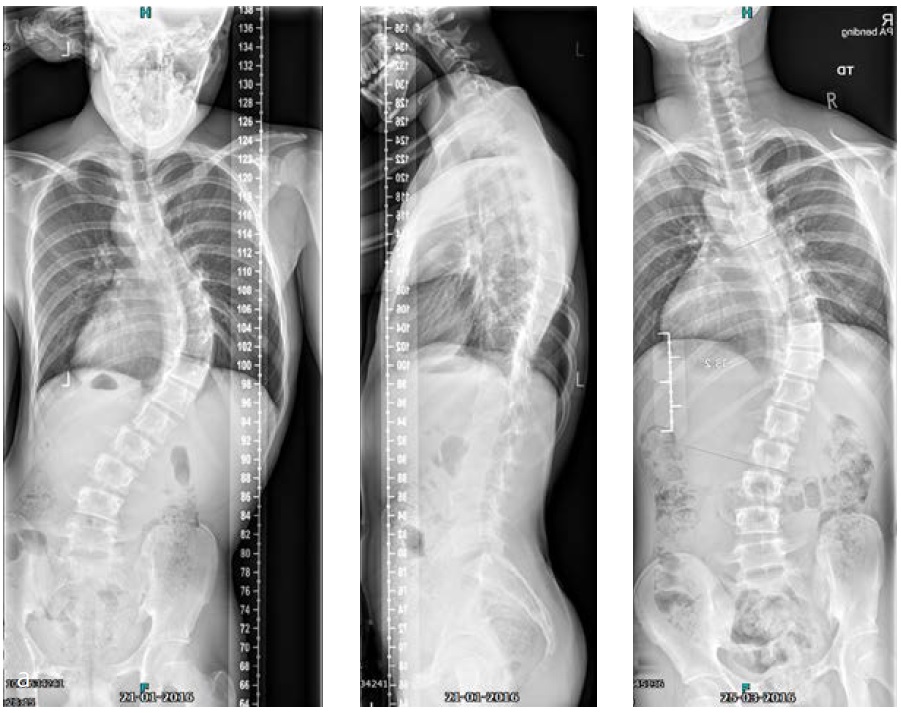
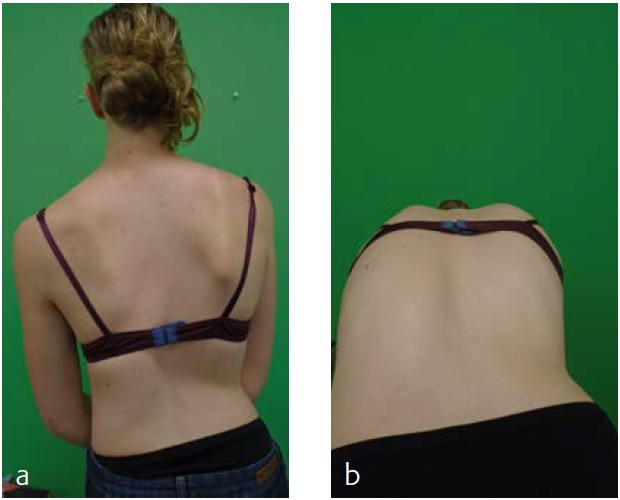
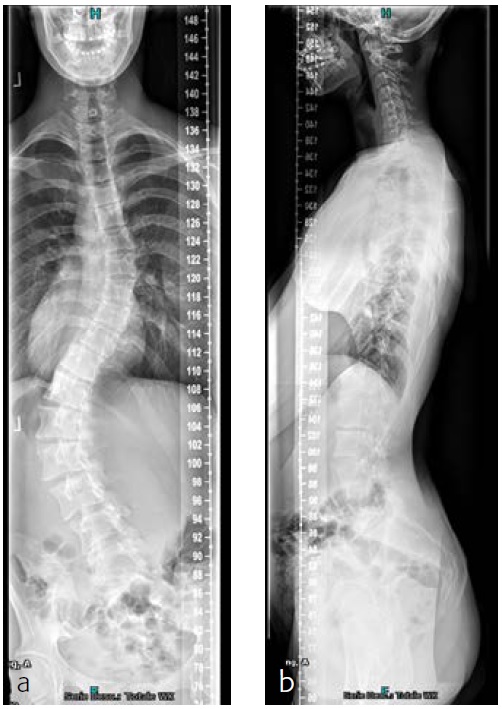
On physical examination a cooperative boy was seen, normal build and height, normal to high paraspinal muscle tone. Standing upright he was off balance to the right. Neurological examination showed absent abdominal skin reflexes bilaterally. The curve was classified as neuromuscular type scoliosis (Fig 6). Due to the curve magnitude, being off balance, and with (severely) limited nonoperative options (Fig 7), surgical treatment was discussed with the family.
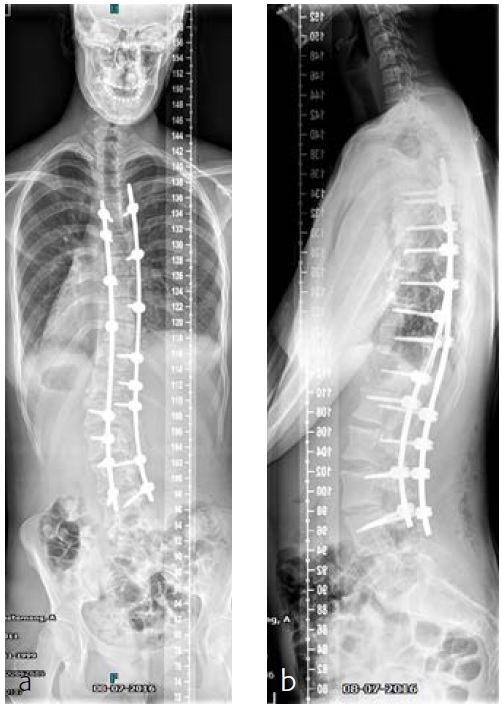
He was scheduled for a posterior deformity correction from T4L3. Under general anesthesia, with IONM (TC-MEP) the deformity was corrected. Intraoperatively, an epidural catheter was placed with the tip at T7 for postoperative analgesia.
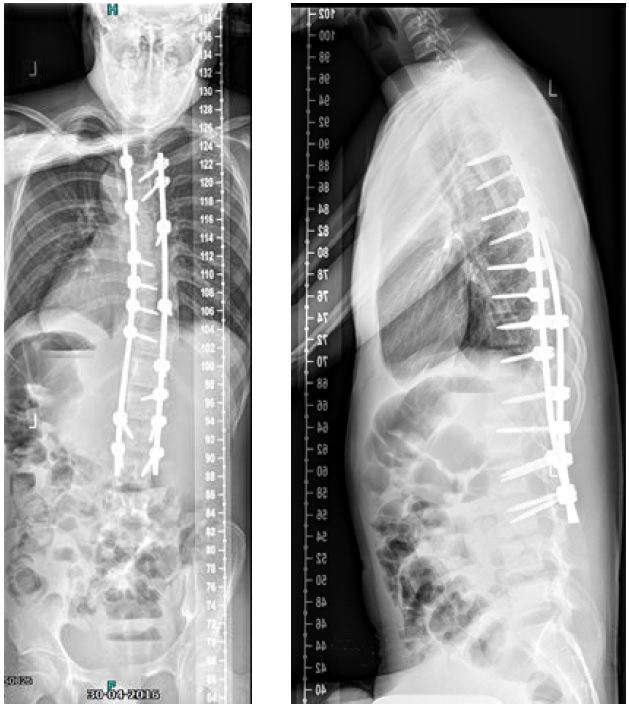
Mobilisation started the day after surgery. He was discharged the fourth day after surgery. He returned for scheduled follow-up after 7 weeks (Fig 8). He seemed less agitated compared to the period before surgery. He did not seem to have specific limitations.
Case 2: 17-year-old female patient
The patient was known and under orthopedic control for a Lenke 5C-type AIS since 2009. Initial treatment with a Boston Brace failed to halt progressive growth, and curve progression became apparent beyond surgical treatment threshold (Fig 9). Bending FS showed TL correction 58 -> 30 (plm 50%). MT correction 43 -> 16 (plm 35%) (Fig 10). The patient was referred to our hospitalfor logistics regarding surgical planning.
After her visit to our clinic, she was planned for surgical correction of the deformity from T5L4 (Fig 11). Surgical procedure with IONM (TC-MEP) postoperative epidural analgesia with the catheter tip at T8. She was mobilised the first postoperative day (Fig 12) and discharged the fourth day after surgery. The patient returned for her 6-month follow-up without any complaints. Limitations are in line with our advice (no sports for 6 months postoperatively). She has no pain and uses no medication.
A novel implant-based solution to spinal deformity correction
Safety and complications associated with cement-augmented pedicle screws―retrospective milestone D report
Ann-Sophie Neuts, MD, Maarten Spruit, MD
Introduction and clinical need
With an aging population there is increasing complexity in the treatment of various spinal problems which demand an operative solution. There is a decrease in bone quality resulting in osteopenia and osteoporosis. This may create associated and expected problems of screw pull out, screw loosening, and proximal junctional kyphosis (PJK) or failure (PJF). Preoperative workup and assessment of bone quality is mandatory [1]. This was recently also addressed by the AO Technical Commission Spine in an Osteoporotic Spine Surgery Task Force and recommendations for preoperative workup in elective spine surgery in patients older than 50 years were presented in a white paper report. Still, prevention of PJK and PJF is an ongoing debate for which the ultimate solution has not yet been found. The aim is always to address sagittal alignment and apply secure instrumentation as well as possible. Longer fusion trajectories seem to increase the risk of failure.
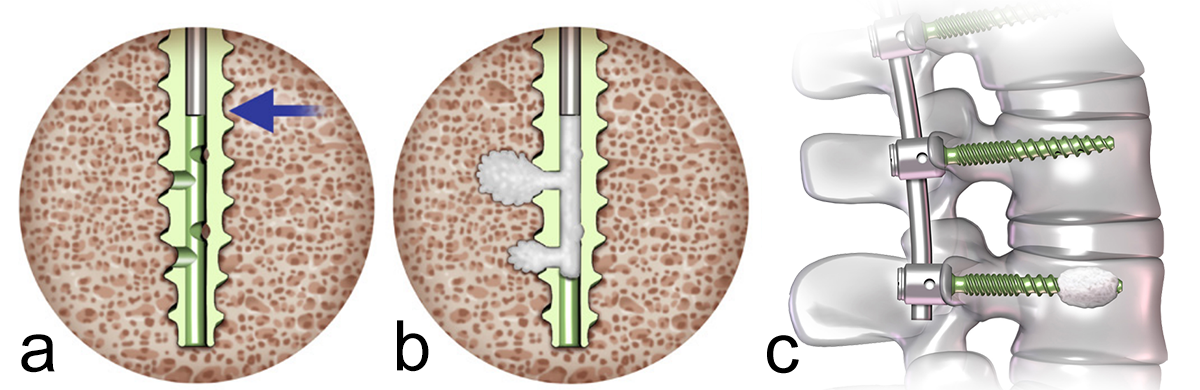
What we know is that cement-augmented pedicle screws (CAPS) can be used to provide additional vertebral bone purchase and fixation. A pedicle screw with good purchase will be a pedicle screw that can correct deformity, reduce slip, and maintain vertebral fixation until solid fusion occurs. In the spinal community there is a consensus that augmented pedicle screws are a good alternative in patients with poor bone quality and provide supplementary anchorage compared with traditional nonaugmented pedicle screws (Fig 1).
The polymethylmethacrylate (PMMA) cement which is used, such as CONFIDENCE™ High Viscosity Spinal Cement (DePuy Synthes), has excellent adhesive properties, augments the screw-bone interface to prevent screw pull-out, and therefore reduces the risk of screw loosening or failure. CAPS are used both in primary cases and in revision surgery.
The use of CAPS may also be associated with certain risks and potential complications. Cement leakage is one of the most common complications related to cement augmentation. The cement can extravasate into the surrounding tissues, including not intended entry into the spinal canal, and hence compromise the spinal cord, cauda and nerve roots, or damage/invade blood vessels. This can result in radiculopathy, neural tissue compression, and even vascular compromise and pulmonary embolism. The incidence of cement leakage varies from 6% to 43%, but fortunately leakage is symptomatic in only 0.6− 1.9% [2, 3]. During the exotherm-curing process of cement, heat is generated. The heat can cause thermal injury to the surrounding tissues, resulting in neurological deficits, such as sensory or motor dysfunction, radicular pain, and bladder or bowel dysfunction. The risk of thermal injury increases with the use of excessive cement volume [3, 4].
Although cement augmentation enhances screw fixation, there is still a risk of screw loosening, screw migration (2.2%), or screw breakage (0.6%) due to mechanical failure caused by cyclic loading and stress [3, 5]. As with any surgical procedure there is also a risk of infection. The presence of cement may complicate the management of infection due to a potential heightened risk of biofilm formation. Infection incidence is estimated to be 1.1−5.7% [3, 6].
Material and results
For this Milestone D report, we reviewed 61 patients who had cement-augmented pedicle screw spine surgery between July 2020 and March 2023. In our hospital we use Expedium Verse Advanced Fenestrated Cortical Fix Polyaxial Screws® (DePuy Synthes). The group included 9 men and 52 women with an average age of 62.5 years (range: 31−83 years). 19 (31%) of the 61 operations were revisions of earlier constructs due to hardware failure or PJK/PJF. The other 42 cases were primary fusions with the need to use cement-augmented screws because of poor bone quality, osteopenia, or osteoporosis. Table 1 shows the trajectory of fusion in primary and revision surgery. Most cases included five or more fusion levels. In 61 patients we used a total of 464 fenestrated screws. Cement augmentation was used in 233 screws.
| Primary Surgery | Revision Surgery | |
| 1 Level | 5 | 3 |
| 2 Level | 4 | 4 |
| 3 Level | 2 | 2 |
| 4 Level | 5 | 2 |
| ≥ 5 Level | 26 | 8 |
Table 1: Trajectory of fusion in primary and revision cases.
Cement leakage
We had four cases (four screws) with cement leakage (1.7%). One patient had an anterior vertebral cortical breach due to placement of the screw. Another sustained a lateral breach of the pedicle wall with leakage into the transverse process. In both patients, leakage was noticed during surgery as we always augment each pedicle screw under image intensifier control. Augmentation was ceased as the moment leakage was observed.
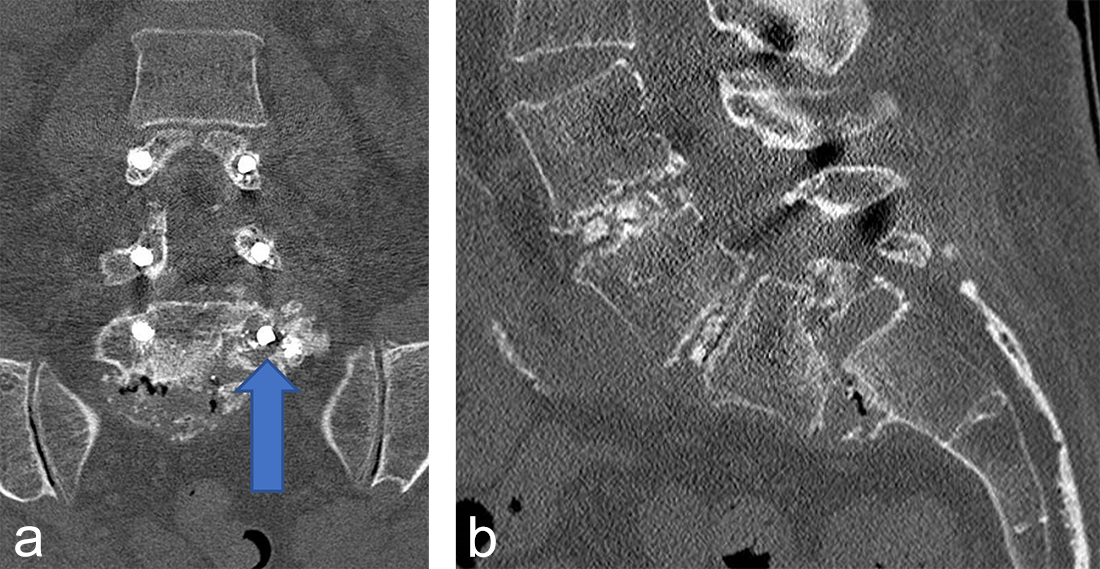
Two other patients had cement leakage into the neuroforamen which caused motor and sensory dysfunction of the affected nerve root. In retrospect the chosen screw length was too short for the vertebra; therefore, the most posterior located perforation in the screw was located within the pedicle (Fig 2).
Other complications
Six (9%) of 61 patients developed a surgical site infection. All had a debridement, antibiotics, and implant retention in the operating room and antibiotic therapy for 3 months. There is extensive variability in literature on the incidence of infection after instrumented spine surgery [7]. Zhou et al [7] described an incidence of 6% in degenerative cases (noninstrumented and instrumented combined). The infection risk is higher in instrumented surgery and the risk increases with prolonged surgery and higher amount of perioperative blood loss. Still, 9% infection rate in our series is relatively high, especially compared with our overall institutional spinal infection rate of 2%. This may be due to multiple revisions in these 6 patients (50%) and the long fusion trajectory with large exposure and longer duration of surgery (33%).
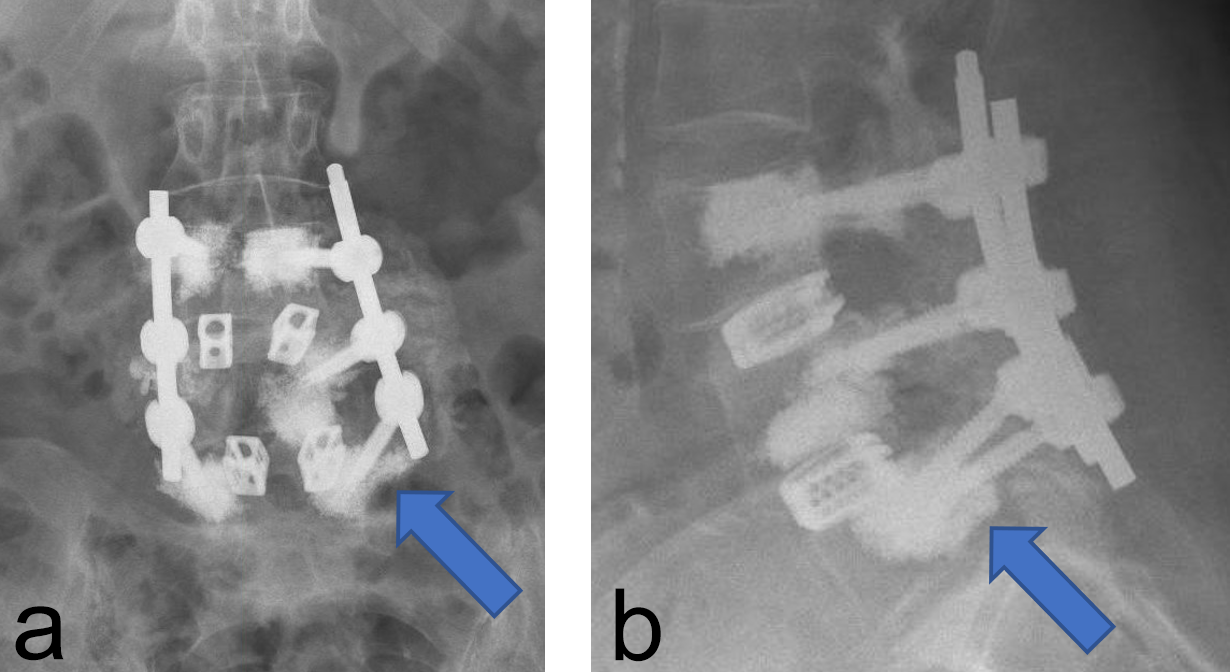
Instrumentation failure occurred in 7 (11.5%) of 61 patients. One patient developed loosening of the three most caudal nonaugmented screws in a long fusion construct. Two patients had screw loosening after a proven infection (one of them with augmented screws). One developed screw loosening due to a pedicle fracture. Another had CAPS loosening after traumatic fracture of the L5 vertebral body (Fig 3). Two patients had a screw breakage. In total 3 (0.86%) of 233 cement- augmented pedicle screws in 61 patients failed despite cement augmentation (Table 2).
| Nonaugmented PS* | CAPS* | |
| Screw loosening | 1 | 0 |
| Infection + screw loosening | 1 | 1 |
| Pedicle No. + screw loosening | 1 | 0 |
| Screw breakage | 1 | 1 |
| Vertebral No. + screw loosening | 0 | 1 |
Table 2: Cases of instrumentation failure in nonaugmented and augmented screws. *PS indicates pedicle screw; CAPS, cement-augmented pedicle screws.
Clinical case
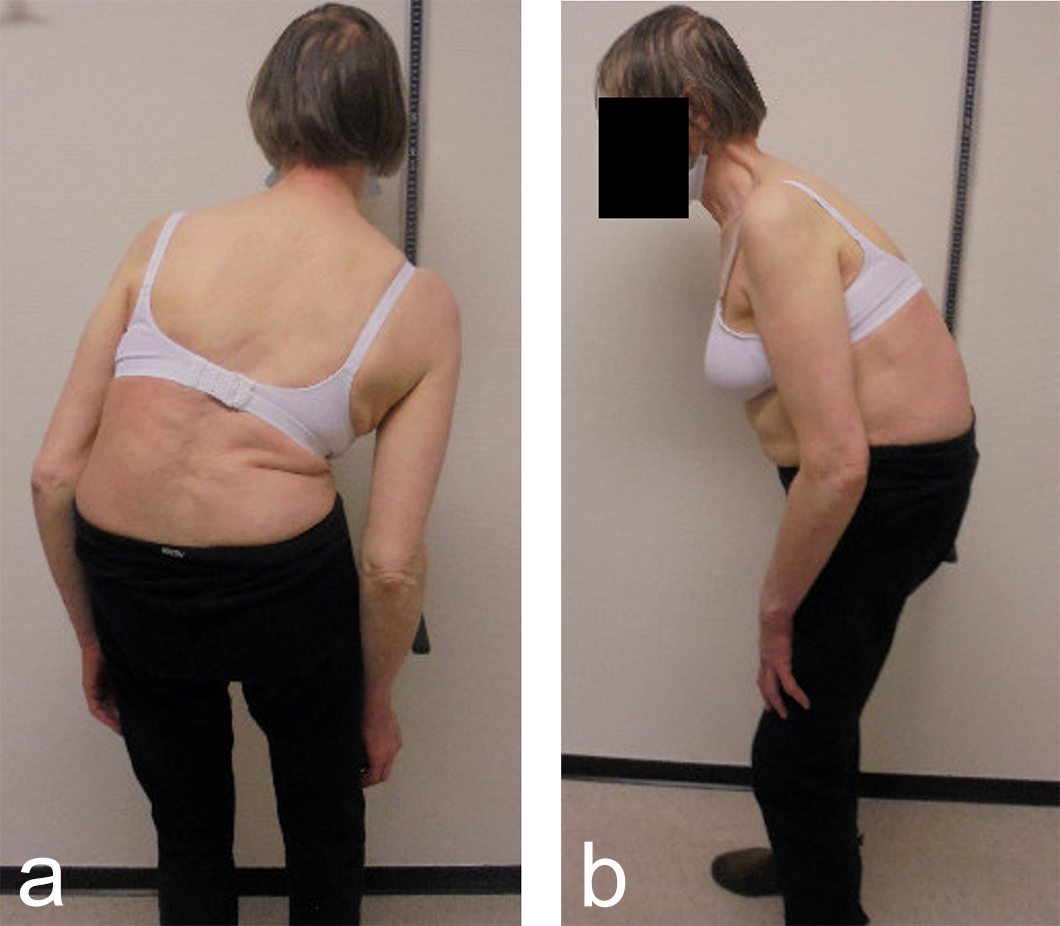
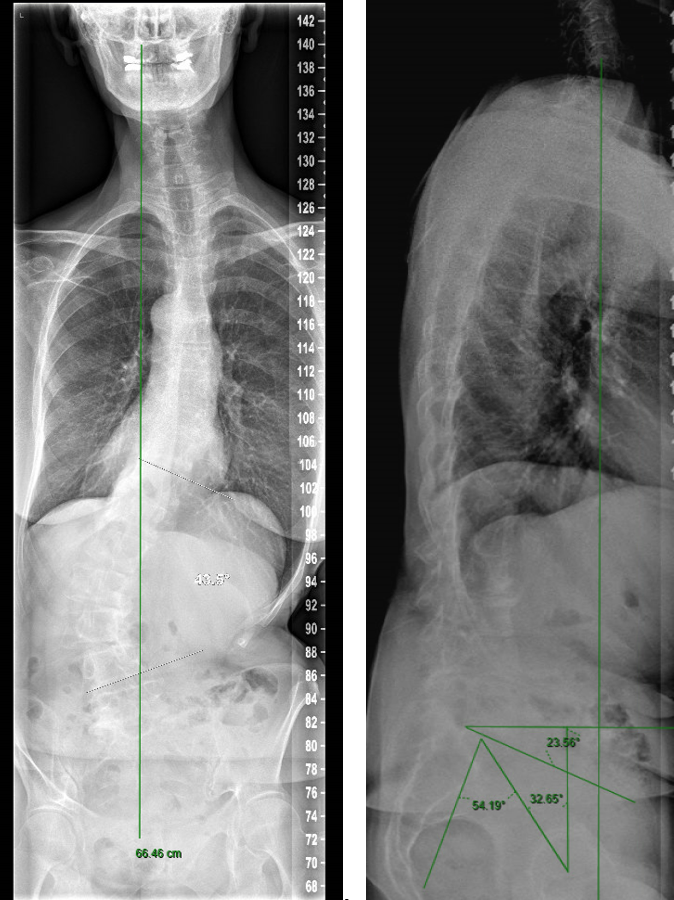
A 70-year-old woman with an adult spinal deformity (ASD) was referred to our hospital for a second opinion. Her medical history included rheumatoid arthritis for which she was given long-term methotrexate therapy and cures of corticosteroids. She presented with a severe degenerative deformity and right L3 radiculopathy. The patient was completely off balance with a right coronal shift and a positive sagittal balance with a SVA of 10 cm+ (Fig 4). Figure 5 reveals full spine images and pelvic parameters. She led a sedentary lifestyle because of the disturbance of balance and back and right leg pain. The patient opted for surgery after a shared decision process and informed consent.
The surgery was planned as a posterior approach to correct the spinal deformity and restore coronal as well as sagittal balance with direct and indirect decompression of the right L3 nerve root. The preoperative bone assessment and DEXA scan indicated osteopenia and considering her medical history the decision was to use cortical fix screws that also allowed cement augmentation.
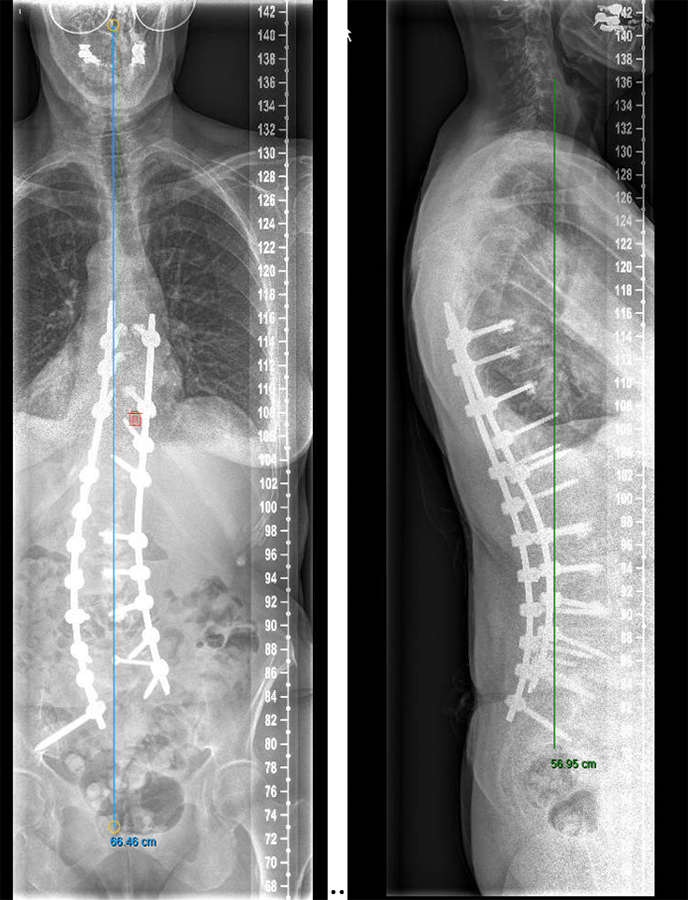
We performed a posterior fusion and correction of Th8-S2-SI-Ilium (Fig 6). In Th9, Th10, Th11, and Th12 we instrumented only unilateral pedicle screws due to small pedicle diameters. A S2-SI-Ilium screw was placed on the left side. A supplemental translaminar screw was used at L5-S1 on the right side. We augmented the screws in Th8, Th9, Th10, L2, L3, and L4. Additionally, a TLIF approach was performed on L2-3 and L3-4 with autogenous bone as interbody support. No complications occurred during nor after surgery, and she remained in good balance during the 1-year postoperative follow-up.
Conclusion
Cement-augmented pedicle screws are effective and safe to use in patients with poor bone quality who require spinal instrumentation. Accurate surgical technique and safe cement augmentation under image intensifier guidance is mandatory. Cement leakage in this Milestone D report is 1.7%, which is low compared with published data. Cement-augmented screw failure is 0.86%. Other complications are not related to this type of instrumentation but to the surgical procedure itself.
References
- Sardar ZM, Kim Y, Lafage V, et al. State of the art: proximal junctional kyphosis diagnosis, management, and prevention. Spine Deform. 2021 May;9(3):635−644.
- Lieberman IH, Hardenbrook MA, Wang JC, et al. Assessment of pedicle screw placement accuracy, procedure time, and radiation exposure using a miniature robotic guidance system. J Spinal Disord Tech. 2012;25(5):241−248.
- Zhang J, Wang G, Zhang N. A meta-analysis of complications associated with the use of cement-augmented pedicle screws in osteoporosis of spine. Orthop Traumatol Surg Res. 2021 Nov;107(7):102791.
- Ledonio CG, Polly DW Jr, Swiontkowski MF. Management of thoracolumbar spine fractures. Spine J. 2014;14(1):145−164.
- Kim CW, Lee YP, Taylor W, et al. Use of polymethylmethacrylate-augmented pedicle screws for the surgical treatment of a thoracolumbar burst fracture: case report. J Neurosurg Spine. 2008;8(3):287−291.
- Lertudomphonwanit T, Goel VK, Traynelis VC, et al. Biomechanical effect of bone cement augmentation on pedicle screw fixation in different quality of bone: a finite element analysis. Spine (Phila Pa 1976). 2016;41(6):E323−E328.
- Zhou J, Wang R, Huo X, et al. Incidence of surgical site infection after spine surgery: a systematic review and meta-analysis. Spine (Phila Pa 1976). 2020 Feb 1;45(3):208−216.
Hazards and labeling
Due to varying countries’ legal and regulatory approval requirements, consult the appropriate local product labeling for approved intended use of the products described on this website. All devices on this website are approved by the AO Technical Commission. For logistical reasons, these devices may not be available in all countries worldwide at the date of publication.
Legal restrictions
This work was produced by AO Foundation, Switzerland. All rights reserved by AO Foundation. This publication, including all parts thereof, is legally protected by copyright.
Any use, exploitation or commercialization outside the narrow limits set forth by copyright legislation and the restrictions on use laid out below, without the publisher‘s consent, is illegal and liable to prosecution. This applies in particular to photostat reproduction, copying, scanning or duplication of any kind, translation, preparation of microfilms, electronic data processing, and storage such as making this publication available on Intranet or Internet.
Some of the products, names, instruments, treatments, logos, designs, etc referred to in this publication are also protected by patents, trademarks or by other intellectual property protection laws (eg, “AO” and the AO logo are subject to trademark applications/registrations) even though specific reference to this fact is not always made in the text. Therefore, the appearance of a name, instrument, etc without designation as proprietary is not to be construed as a representation by the publisher that it is in the public domain.
Restrictions on use: The rightful owner of an authorized copy of this work may use it for educational and research purposes only. Single images or illustrations may be copied for research or educational purposes only. The images or illustrations may not be altered in any way and need to carry the following statement of origin “Copyright by AO Foundation, Switzerland”.
Check www.aofoundation.org/disclaimer for more information.
If you have any comments or questions on the articles or the new devices, please do not hesitate to contact us.
“approved by AO Technical Commission” and “approved by AO”
The brands and labels “approved by AO Technical Commission” and “approved by AO”, particularly "AO" and the AO logo, are AO Foundation's intellectual property and subject to trademark applications and registrations, respectively. The use of these brands and labels is regulated by licensing agreements between AO Foundation and the producers of innovation products obliged to use such labels to declare the products as AO Technical Commission or AO Foundation approved solutions. Any unauthorized or inadequate use of these trademarks may be subject to legal action.
AO ITC Innovations Magazine
Find all issues of the AO ITC Innovations Magazine for download here.
Innovation Awards
Recognizing outstanding achievements in development and fostering excellence in surgical innovation.