Anterior Cervical Interbody Spacer
Anterior Cervical Interbody Spacer (ACIS)
The ACIS implant is designed to meet the specific demands of anterior cervical interbody fusion procedures, as described in various publications [1-6].
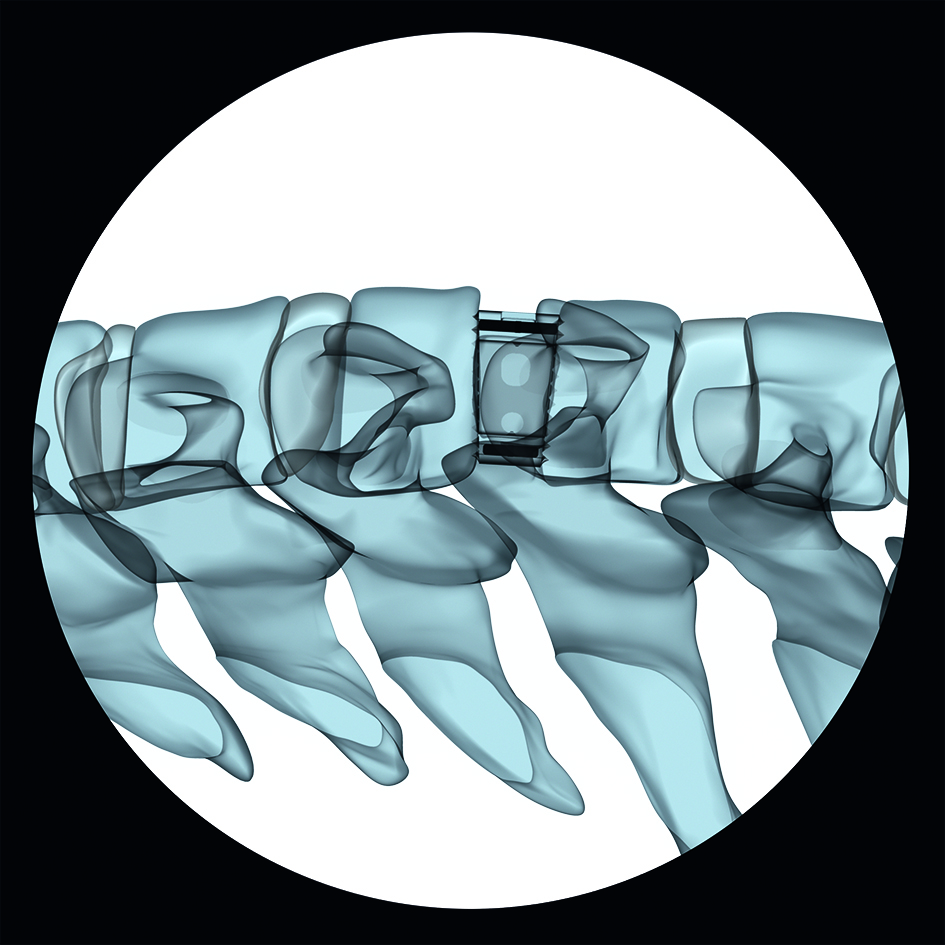
In order to enable visualization of the implant position, the implant possesses three radiographic marker pins as shown in Figure 1 and 2.
Intended use
The ACIS system is intended to replace cervical intervertebral discs and to fuse adjacent vertebral bodies at vertebral levels C2-C7 following anterior cervical discectomy for reduction and stabilization of the cervical spine. The use of autologous bone or bone graft substitute is recommended.
Indications:
Cervical pathologies for which segmental arthrodesis is indicated:
- Degenerative disc diseases and instabilities
- Ruptured and herniated discs
- Pseudarthrosis or failed spondylodesis
For multisegmental fusions with the ACIS system supplemental fixation is recommended.
Contraindications:
- Osteoporosis
- Severe instabilities
- Vertebral body fractures
- Spinal tumors
- Infections
References
1) Barsa P, Suchomel P. Factors affecting sagittal malalignment due to cage subsidence in standalone cage assisted anterior cervical fusion. Eur Spine J. 2007;16(9):1395-1400.
2) Caspar W, Geisler FH, Pitzen T, et al. Anterior cervical plate stabilization in one- and two-level degenerative disease: overtreatment or benefit? J Spinal Disord. 1998;11(1):1-11.
3) Fraser JF, Hartl R. Anterior approaches to fusion of the cervical spine: a metaanalysis of fusion rates. J Neuro Spine. 2007 Apr;6(4):298-303.
4) Kaiser MG, Haid RW Jr, Subach BR, et al. Anterior cervical plating enhances arthrodesis after discectomy and fusion with cortical allograft. Neurosurg. 2002 Feb;50(2):229-236.
5) Mobbs RJ, Rao P, Chandran NK. Anterior cervical discectomy and fusion: analysis of surgical outcome with and without plating. J Clin Neuroscience. 2007 Jul;14(7):639-642.
6) Moftakhar R, Trost GR. Anterior cervical plates: a historical perspective. Neurosurgical Focus. 2004 Jan;16(1):E8.
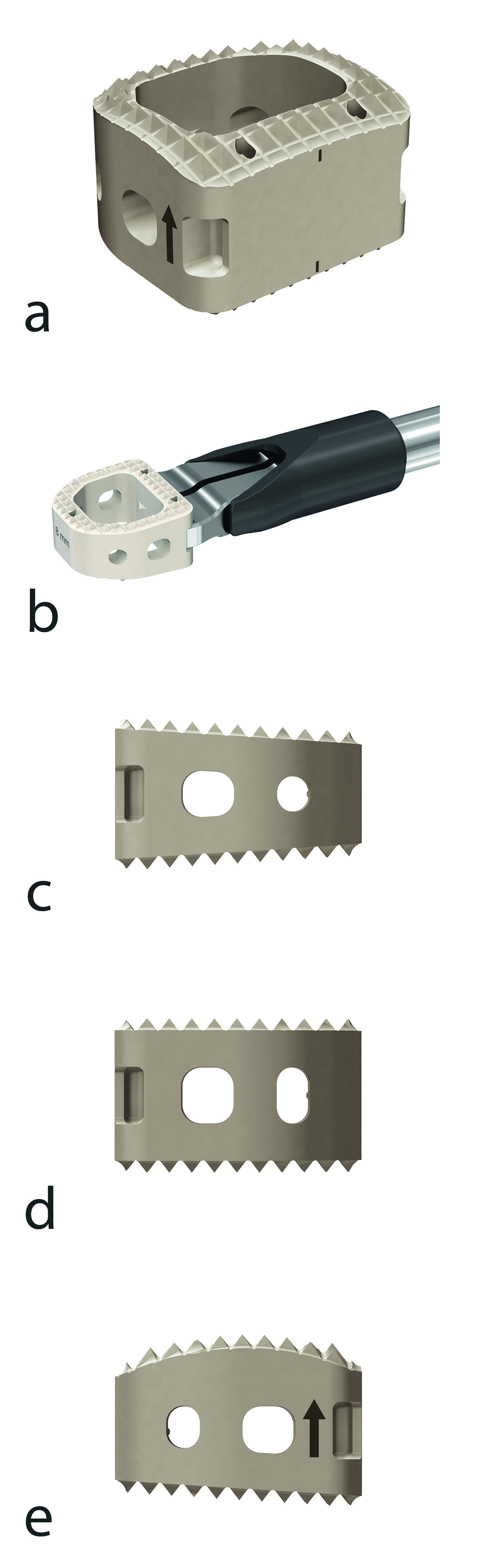
The implant features a large central canal and is available in three different sagittal shapes (convex, parallel, and lordotic) and comes in 3 footprint sizes (small, ie, 11.5 mm 12.5 mm, standard, ie, 13 mm 14 mm, and large, ie, 15 mm 16 mm) and 8 heights ranging from 5 mm to 12 mm in 1 mm increments to accommodate various patient anatomies.
Pyramidal teeth provide resistance to implant migration, whereas the large central canal accommodates autogenous bone graft or other bone graft substitute to allow fusion to occur through the implant (Fig 3).
Anterior Cervical Interbody Spacer (ACIS): Focused Registry study
Degenerative cervical spine conditions can lead to chronic neck and arm pain. Surgical treatment may require anterior decompression and fusion, which is usually established with a cage implant. A recent systematic review described the evolution of cervical cage materials [1]. It noted that the evolution of cage materials had accompanied the changes in design and found that three materials had primarily been used in the manufacture of cage implants:
- Carbon fiber reinforced polymers (CF-P)
- Titanium (Ti)
- Polyetheretherketone (PEEK).
The study found that when initially trialed, CF-P cages achieved high rates of fusion and good to excellent clinical outcomes, however, they have largely been superseded by PEEK due to its superior elastic modulus. Titanium and its alloys were one of the first materials to be utilized for cages in the 1980s. Used by the orthopedic world since the 1940s, Ti is a robust biomaterial with excellent corrosion resistance and a low density that can undergo surface modification to improve osseointegration and cell adhesion. PEEK cages were introduced in the 1990s as an alternative to Ti cages and provide the advantages of radiolucency and an elastic modulus close to bone, thereby avoiding the stress shielding associated with Ti [1].
Today, controversy exists regarding the use of Ti versus PEEK cages [1]. Although PEEK has theoretical advantages, this has not clearly transferred into the clinical setting due to the difficulty in determining and controlling for other surgical factors, including the roles of endplate preparation, area of contact, and overdistraction. However, the majority of studies have reported improved fusion rates, lower subsidence rates, and radiolucency with PEEK versus Ti cages, with one long-term study reporting limited differences in the early postoperative period, but better maintenance of intervertebral height, cervical lordosis, and clinical outcomes by PEEK cages by the 7-year follow-up [2].
Thus, it seems that PEEK cages are the most promising recent development in the field. The Anterior Cervical Interbody Spacer (ACIS), which was the subject of this study, is made from PEEK and has only recently been made commercially available. It can be used as a standalone implant or with additional anterior plate fixation. The choice to add a plate or not, as well as the choice of the filling material (bone or bone substitute), was left to the surgeons discretion.
An AO focused registry was conducted in accordance with the ethical principles set forth in the Declaration of Helsinki (DoH) including amendments as well as the International Conference of Harmonization Good Clinical Practice (ICH GCP) guidelines, the European Standard EN ISO14155/20032011, and the laws and regulations of the individual countries in which the research was conducted.
Title of the clinical study
AO Spine TK ACIS registry-A Focused Registry on Anterior Cervical Interbody Spacer procedures in patients with cervical spine degeneration (Clinical trial registry number NCT02016794).
Purpose of the clinical study
The purpose of the study was to document the clinical experience of a new PEEK cage (ACIS) used for anterior cervical decompression and fusion (ACDF) by prospectively collecting (a) clinical data on pain, function, activities of daily living (ADL), adverse events, and length of hospital stay, (b) radiological outcomes, eg, fusion status, and (c) implant handling data.
Study population, design, and dates
Patients with a degenerative cervical condition that required anterior decompression and fusion on single or multiple levels. Prospective observational case series. Clinical study initiation date: first patient March 26, 2015. Clinical study completion date: the last follow-up was carried out January 12, 2016.
Results of the clinical study
This focused registry of the Anterior Cervical Interbody Spacer (ACIS) included nine patients. For less than half of the included patients, information was collected immediately postoperatively. Based on the small number of patients, the generalizability of the study results is overall limited. This particularly applies to the information collected immediately postoperatively, which will not be considered for result interpretation.
The primary outcome measure of this study was the Neck Disability Index (NDI) after implantation of ACIS. A distinct improvement of the mean NDI from 18.4 (SD= 8.7) preoperatively to 8.0 (SD = 6.2) 6 months after surgery was seen as time after surgery progressed. Secondary outcomes were pain for neck and arms using a Numeric Rating Scale (NRS) ranging from 0 for no pain to 10 for worst imaginable pain, implant handling details, length of hospital stay, adverse events, and radiological outcomes.
Both neck and arm pain NRSs improved steadily with time after surgery from a preoperative mean of 5.4 (SD = 2.3) to a mean of 2.8 (SD = 2.4) at 6 months for neck pain and from a preoperative mean of 4.7 (SD = 3.6) to a mean of 0.9 (SD = 1.1) at 6 months for arm pain.
A wide range of implant handling aspects was documented, including endplate preparation with endplate rasp, trialing with trial implants, packing bone into the implant, implant insertion with insertion device, implant insertion with implant holder, implant insertion with ACF holder, and overall ease of use. Each of these aspects were either rated as easy or very easy by the investigators. None of the handling aspects was rated as moderate, difficult or very difficult. All investigators would use ACIS again for this application.
The mean length of hospital stay was 3 days (SD=0.9). No adverse events were reported within the 6 months timeframe of the study, however, in one patient, cage subsidence was recorded as an adverse event 235 days
after surgery. The patient was reported to have recovered without any persistent damage.
Upon radiological evaluation, fusion was determined in 56% of patients (5/9). The x-rays of two patients were not assessable and two patients were evaluated as having pseudarthrosis. The reason for pseudarthrosis was subsidence in one patient and radiolucency around the cage in the other patient.
Conclusions
Even though the limited number of patients does not permit us to draw final conclusions on the clinical performance of the implant, the fact that no adverse events were reported in the 6 months timeframe of the study and that no reoperations had to be performed in the study population is highly encouraging. The improvement in pain and function as well as the positive feedback with regard to implant handling further adds to the positive impression.
References
1) Chong E, Pelletier MH, Mobbs RJ, et al. The design evolution of interbody cages in anterior cervical discectomy and fusion: a systematic review. BMC Musculoskeletal Disorders. 2015;16:99.
2) Chen Y, Wang X, Lu X, et al. Comparison of titanium and polyetheretherketone cages in the surgical treatment of multilevel cervical spondylotic myelopathy. Eur Spine J. 2013;22(7):1539-1546.
Hazards and labeling
Due to varying countries’ legal and regulatory approval requirements, consult the appropriate local product labeling for approved intended use of the products described on this website. All devices on this website are approved by the AO Technical Commission. For logistical reasons, these devices may not be available in all countries worldwide at the date of publication.
Legal restrictions
This work was produced by AO Foundation, Switzerland. All rights reserved by AO Foundation. This publication, including all parts thereof, is legally protected by copyright.
Any use, exploitation or commercialization outside the narrow limits set forth by copyright legislation and the restrictions on use laid out below, without the publisher‘s consent, is illegal and liable to prosecution. This applies in particular to photostat reproduction, copying, scanning or duplication of any kind, translation, preparation of microfilms, electronic data processing, and storage such as making this publication available on Intranet or Internet.
Some of the products, names, instruments, treatments, logos, designs, etc referred to in this publication are also protected by patents, trademarks or by other intellectual property protection laws (eg, “AO” and the AO logo are subject to trademark applications/registrations) even though specific reference to this fact is not always made in the text. Therefore, the appearance of a name, instrument, etc without designation as proprietary is not to be construed as a representation by the publisher that it is in the public domain.
Restrictions on use: The rightful owner of an authorized copy of this work may use it for educational and research purposes only. Single images or illustrations may be copied for research or educational purposes only. The images or illustrations may not be altered in any way and need to carry the following statement of origin “Copyright by AO Foundation, Switzerland”.
Check www.aofoundation.org/disclaimer for more information.
If you have any comments or questions on the articles or the new devices, please do not hesitate to contact us.
“approved by AO Technical Commission” and “approved by AO”
The brands and labels “approved by AO Technical Commission” and “approved by AO”, particularly "AO" and the AO logo, are AO Foundation's intellectual property and subject to trademark applications and registrations, respectively. The use of these brands and labels is regulated by licensing agreements between AO Foundation and the producers of innovation products obliged to use such labels to declare the products as AO Technical Commission or AO Foundation approved solutions. Any unauthorized or inadequate use of these trademarks may be subject to legal action.
AO ITC Innovations Magazine
Find all issues of the AO ITC Innovations Magazine for download here.
Innovation Awards
Recognizing outstanding achievements in development and fostering excellence in surgical innovation.