SYNTHECEL Dura Repair
Christian Matula
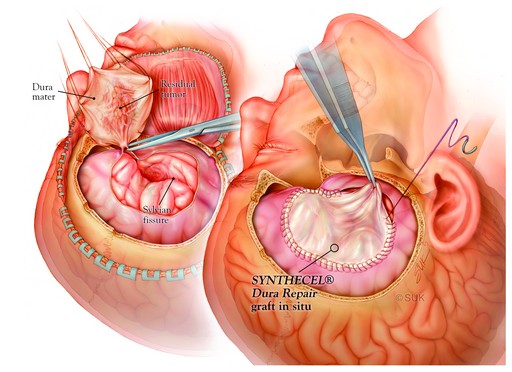
SYNTHECEL Dura Repair Dura Repair is a dural substitute based on biosynthesized cellulose technology. It is designed for the repair of dura mater during cranial or spinal surgery, following traumatic, neoplastic or inflammatory damage.
Unmet clinical needs in dural repair
Materials currently used for dura replacement include human tissues (e.g. pericranium or fascia lata), animal tissues, polymers and biosynthetic substances. However, use of these materials can be problematic. Autologous tissue grafts can perform well as they do not provoke inflammatory or immunological reactions, but can present difficulties in achieving watertight closure, formation of scar tissue, a graft material to close large dural defects and morbidity at the harvest site. Synthetics have been associated with deep wound infections, as polymers may become chronically colonized. Xenografts may cause adverse effects such as graft dissolution, encapsulation, foreign body reaction, scarring and the formation of adhesions. The development of adhesions between dura mater, cortex, temporalis muscle and galea following decompressive craniectomy can act as epileptic foci as well as increasing the surgical risk of subsequent cranioplasty. Additionally, xenografts have been associated with the transmission of viral infections and hydrodynamic complications including persistent CSF leakage, pseudomeningocele, aseptic meningitis and delayed hydrocephalus.
To function effectively, dural substitutes should:
- Prevent CSF leakage
- Minimise risk of infection
- Have mechanical properties similar to human dura and good intra-operative handling properties
- Have no harmful foreign body reaction
- Be readily available
- Be storable
- Be biocompatible
Development of SYNTHECEL Dura Repair
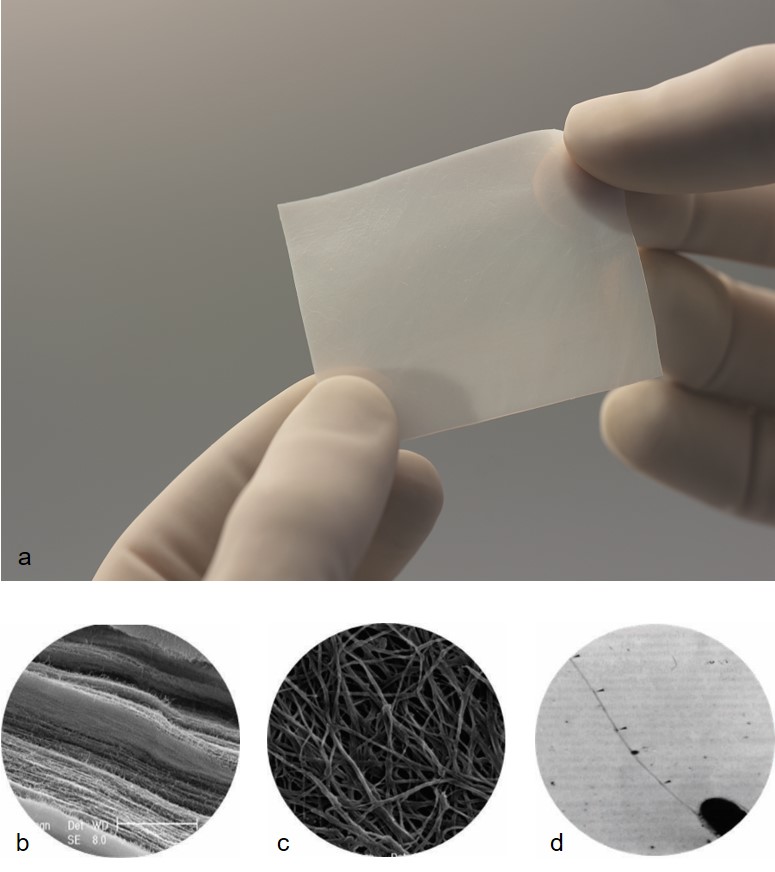
SYNTHECEL was developed as a superior dural substitute, with the aim to eliminate or reduce many of the adverse events associated with materials currently used for dura replacement (see "Details" below). SYNTHECEL Dura Repair is an implant based on biosynthesized cellulose technology. Cellulose pellicles of specified weight and cellulose content are produced by the bacterium Glucoacetobacter xylinus when propagated in nutritive culture media.
Comprised of non-woven, interconnected cellulose fibres, SYNTHECEL has excellent tensile strength and functions as a mechanical layer to protect and repair dural defects whilst preventing CSF leakage (Fig 3). SYNTHECEL is immunologically inert, allows healing without adhesion formation and avoids the complications inherent to the use of autologous tissue in duraplasty. SYNTHECEL is non-animal derived, meaning there is no risk of transmissible diseases.
Clinical Performance of SYNTHECEL Dura Repair
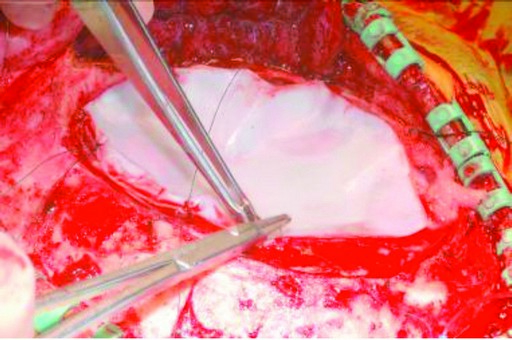
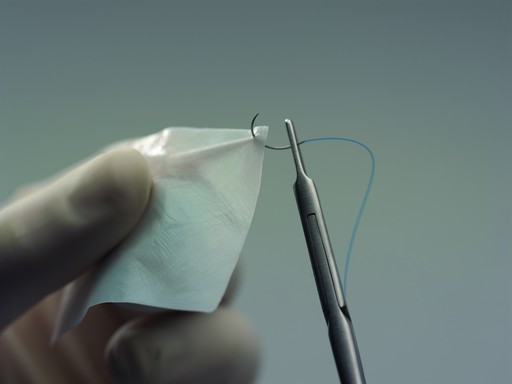
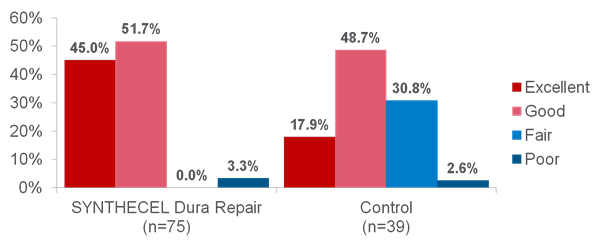
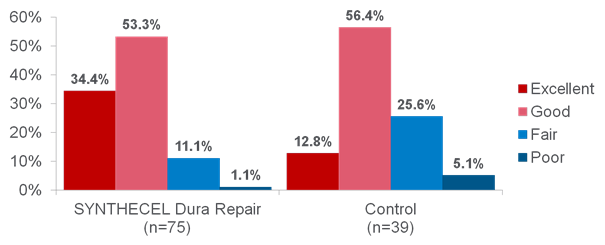
Clinical studies have shown that SYNTHECEL Dura Repair is not inferior to other commercially available dural replacement products in terms of surgical site infection, wound healing assessment or radiologic endpoints (absence of pseudomeningocele and CSF fistula) (1). Furthermore, SYNTHECEL was shown to be superior in terms of product strength, sutureability and seal quality (Fig 4). Indeed, a prospective randomized controlled study (1) found SYNTHECEL to exhibit superior strength and sutureability (Figs 5-6). In terms of surgical handling, SYNTHECEL is similar in thickness to human dura and conforms easily to the brain.
References
(1) Rosen, Charles L., et al. Results of the Prospective, Randomized, Multicenter Clinical Trial Evaluating a Biosynthesized Cellulose Graft for Repair of Dural Defects. Neurosurgery. 2011 Nov;69(5):1093-103; discussion 1103-4. (See Supporting Documents)
Innovative Dural Repair
Prospective, Randomized, Multicenter Clinical Trial Evaluating a Biosynthesized Cellulose Graft for Repair of Dural Defects
Hazards and labeling
Due to varying countries’ legal and regulatory approval requirements, consult the appropriate local product labeling for approved intended use of the products described on this website. All devices on this website are approved by the AO Technical Commission. For logistical reasons, these devices may not be available in all countries worldwide at the date of publication.
Legal restrictions
This work was produced by AO Foundation, Switzerland. All rights reserved by AO Foundation. This publication, including all parts thereof, is legally protected by copyright.
Any use, exploitation or commercialization outside the narrow limits set forth by copyright legislation and the restrictions on use laid out below, without the publisher‘s consent, is illegal and liable to prosecution. This applies in particular to photostat reproduction, copying, scanning or duplication of any kind, translation, preparation of microfilms, electronic data processing, and storage such as making this publication available on Intranet or Internet.
Some of the products, names, instruments, treatments, logos, designs, etc referred to in this publication are also protected by patents, trademarks or by other intellectual property protection laws (eg, “AO” and the AO logo are subject to trademark applications/registrations) even though specific reference to this fact is not always made in the text. Therefore, the appearance of a name, instrument, etc without designation as proprietary is not to be construed as a representation by the publisher that it is in the public domain.
Restrictions on use: The rightful owner of an authorized copy of this work may use it for educational and research purposes only. Single images or illustrations may be copied for research or educational purposes only. The images or illustrations may not be altered in any way and need to carry the following statement of origin “Copyright by AO Foundation, Switzerland”.
Check www.aofoundation.org/disclaimer for more information.
If you have any comments or questions on the articles or the new devices, please do not hesitate to contact us.
“approved by AO Technical Commission” and “approved by AO”
The brands and labels “approved by AO Technical Commission” and “approved by AO”, particularly "AO" and the AO logo, are AO Foundation's intellectual property and subject to trademark applications and registrations, respectively. The use of these brands and labels is regulated by licensing agreements between AO Foundation and the producers of innovation products obliged to use such labels to declare the products as AO Technical Commission or AO Foundation approved solutions. Any unauthorized or inadequate use of these trademarks may be subject to legal action.
AO ITC Innovations Magazine
Find all issues of the AO ITC Innovations Magazine for download here.
Innovation Awards
Recognizing outstanding achievements in development and fostering excellence in surgical innovation.