Facet Wedge Spine System
Frank Kandziora and Maarten Spruit
The intended use, indications and contraindications for FW fixation are very similar to TFS fixation.
Indications
- Stand-alone (bilateral) in situ facet fusion with or without decompression
- Facet arthritis: fixation and fusion of facet joint
- Supplementary fixation after anterior cage or nonunion of ALIF
- Supplementary contra lateral fixation after MISS TLIF
Contraindications
- Unilateral application, except in combination with pedicle screw fixation on the contralateral side
- Compromised facets due to decompression techniques
- Spondylolisthesis
- Fracture or other instabilities of the posterior elements
- Tumor
- Acute or chronic systemic or localized spinal infections
Tips for safety and effectiveness
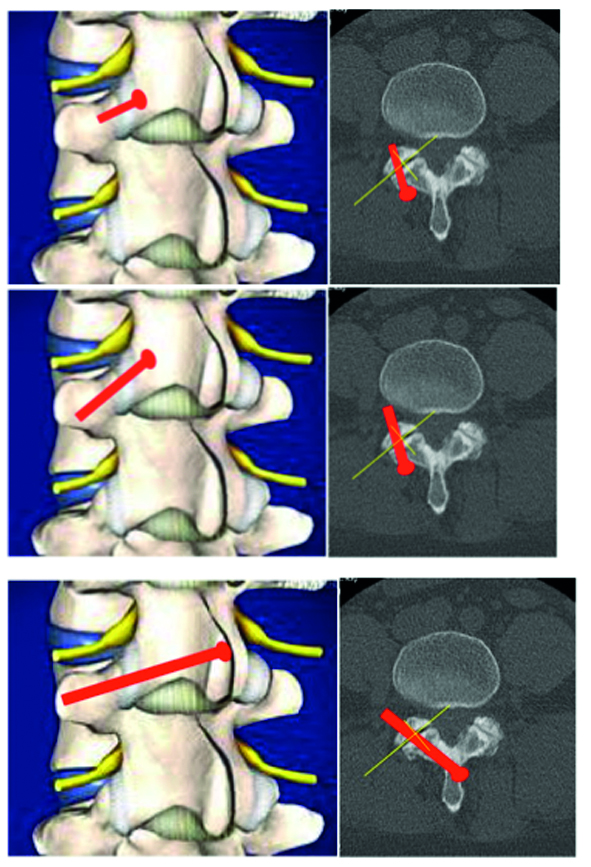
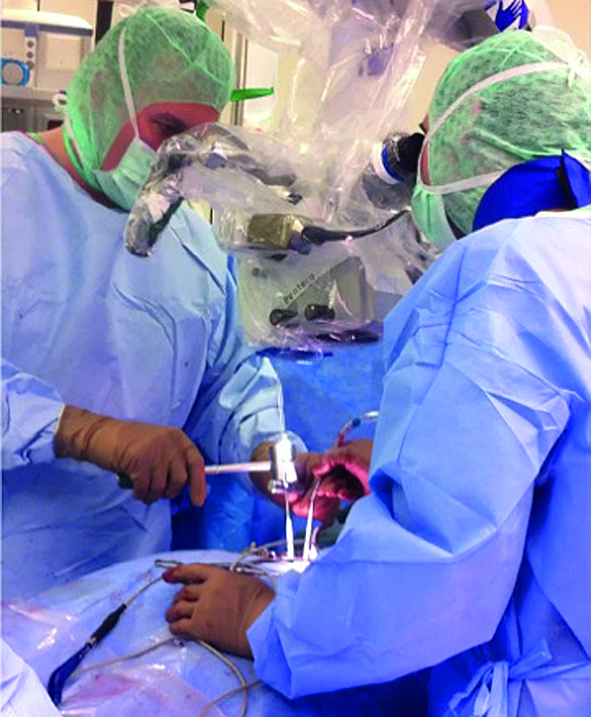
The FW Spine System Risk Assessment identified that incorrect placement of the K-wire for rasp or FW positioning could result in damage to soft tissue, neural structures, or large blood vessels. A second risk involves the use of the facet opener. Excessive force or inappropriate manipulation may also lead to the damage of neural structures. Several control measures are incorporated into the Facet Wedge system to minimize these risks and plans are also in place to conduct a study that will measure their occurrence.
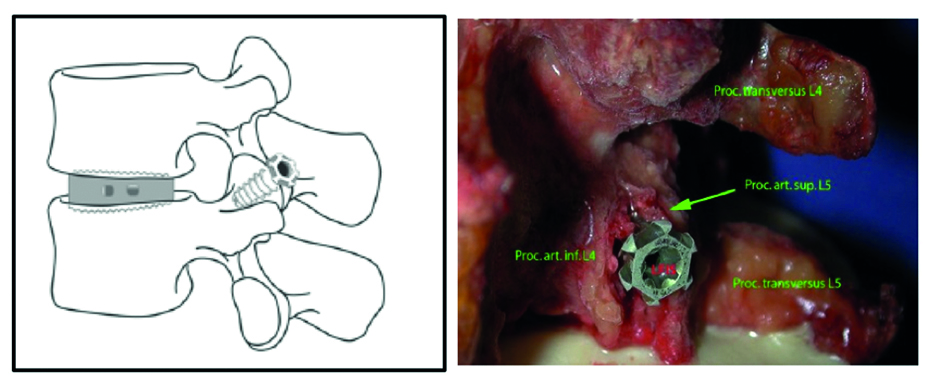
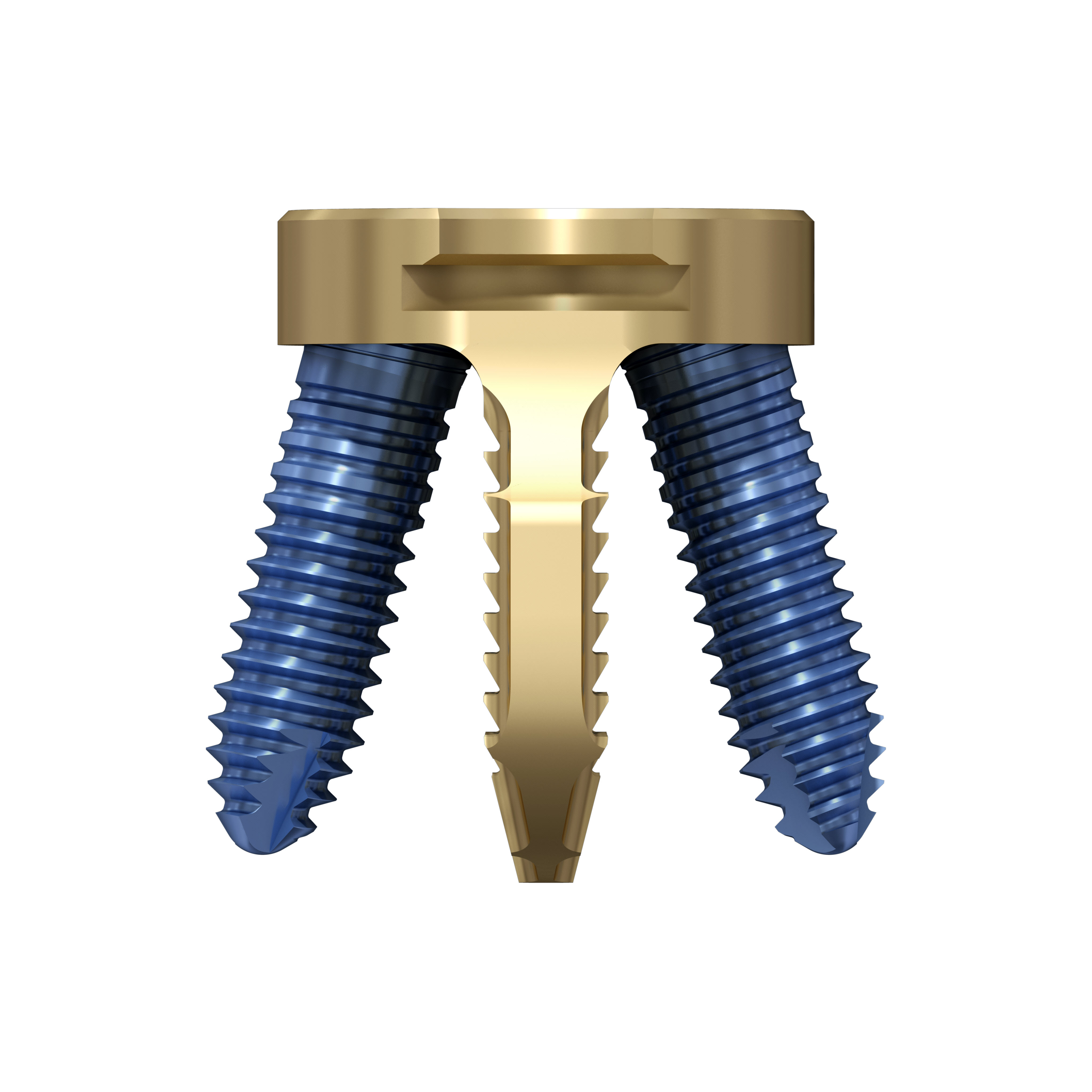
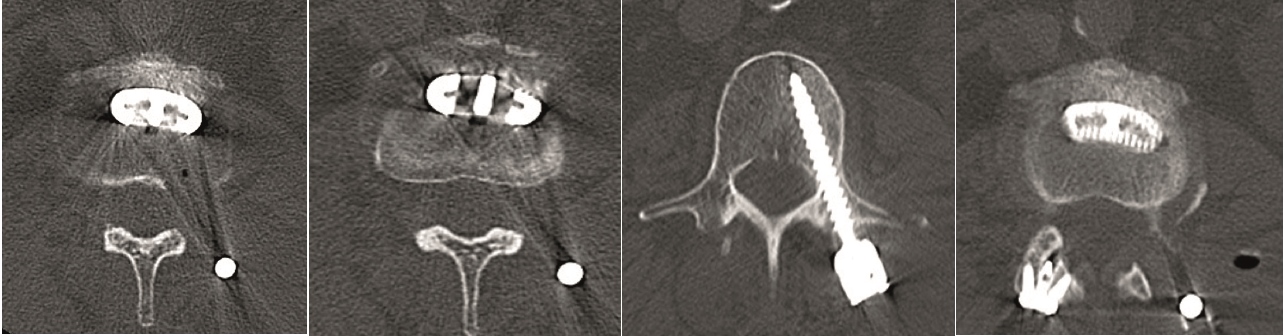
The Facet Wedge spine system includes the following implants and features (Fig 5):
Kirschner wire hole enables guided insertion over K-wire (a)
Rails stop translational motion and generate contact between subchondral bone and implant (a)
Low profile feature decreases muscle irritation (b)
Implant shoulder that controls insertion depth (b)
Teeth keep the implant in the desired position prior to screw insertion (b)
Divergent angular stable locking screws for primary fixation (b)
Various implant sizes to accommodate patient anatomy (b)
Perforations create optimal fusion conditions (c).
Cases provided by Frank Kandziora, Frankfurt, Germany
Case 1
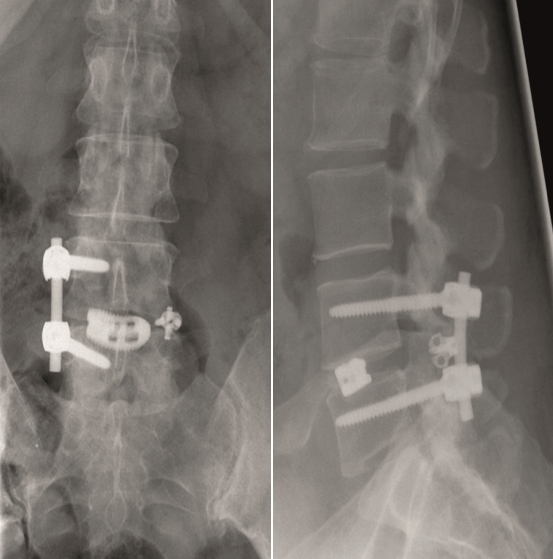
A 45-year-old healthy male patient experienced load dependent lower back pain (LBP) for 6 years, with no radicular pain and no neurologic deficit.
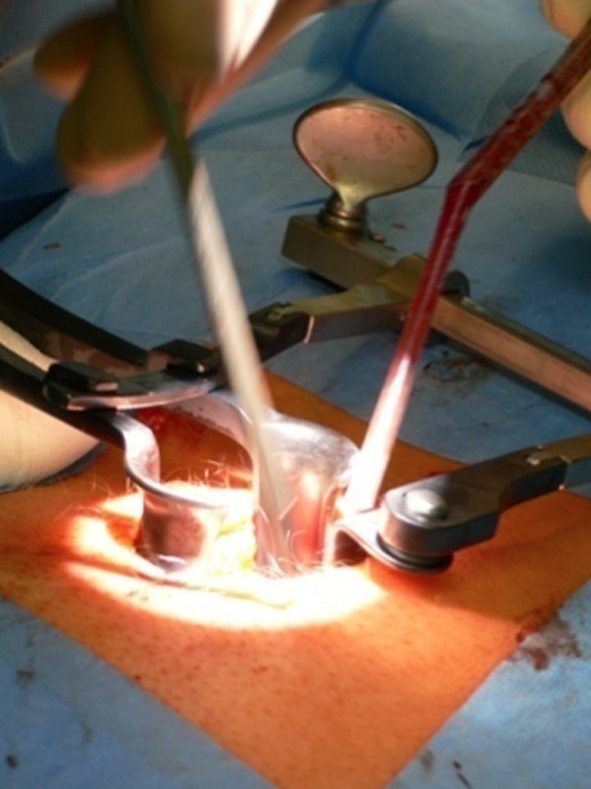
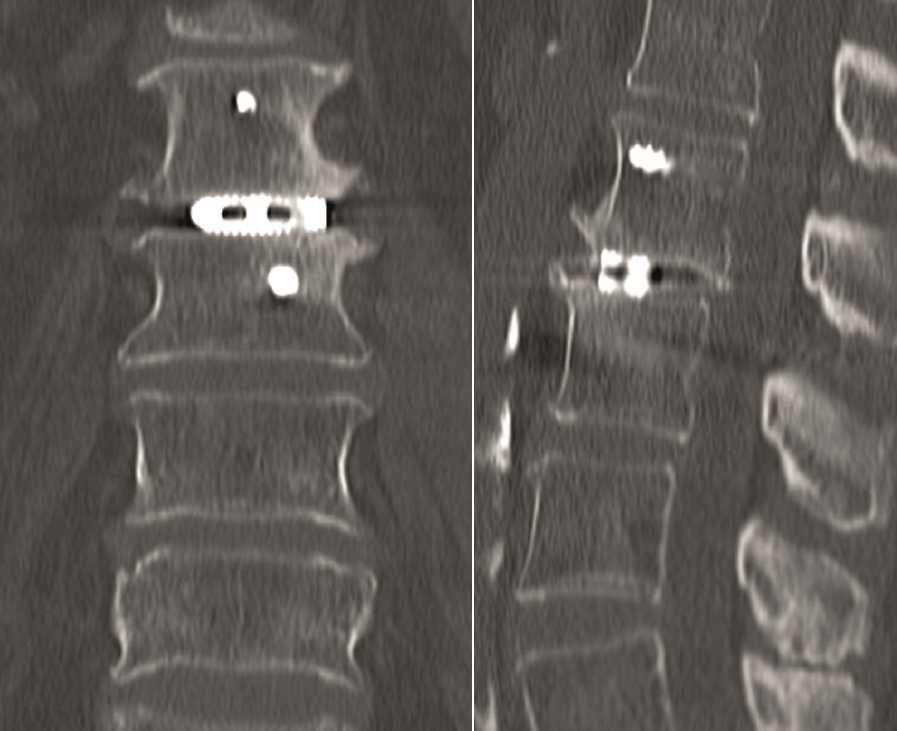
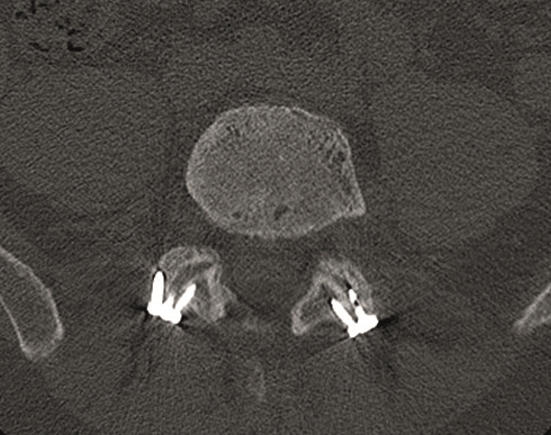
Multilevel facet pathology is shown in Fig 6. Intraoperative and postoperative images are shown (Fig 7-9).
Case 2
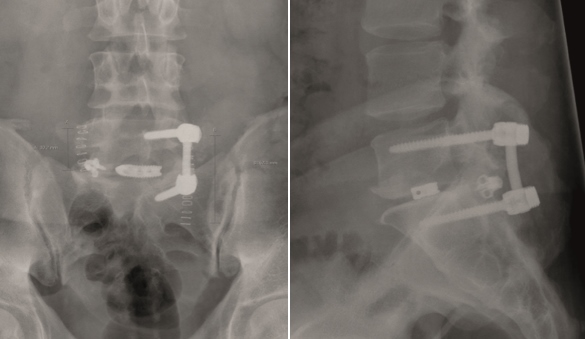
A 51-year-old female patient experiencing LBP for 3 years (Fig 10).
Case 3
A healthy 66-year-old female patient had been experiencing LBP for 5 years.
Cases provided by Maarten Spruit, Nijmegen, Netherlands
Case 4 : ALIF L4-L5 non union
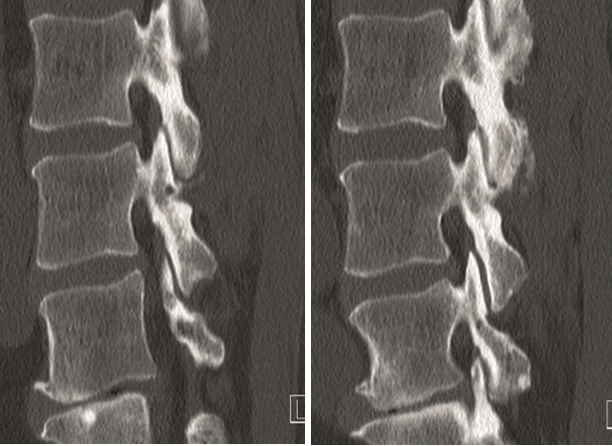
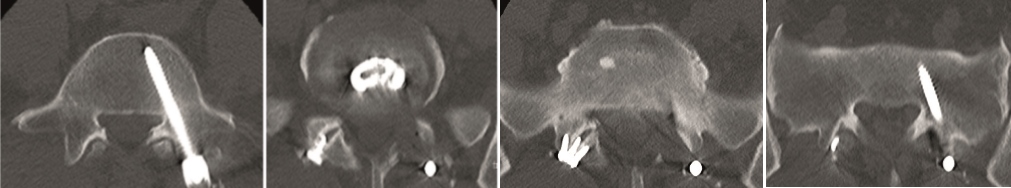
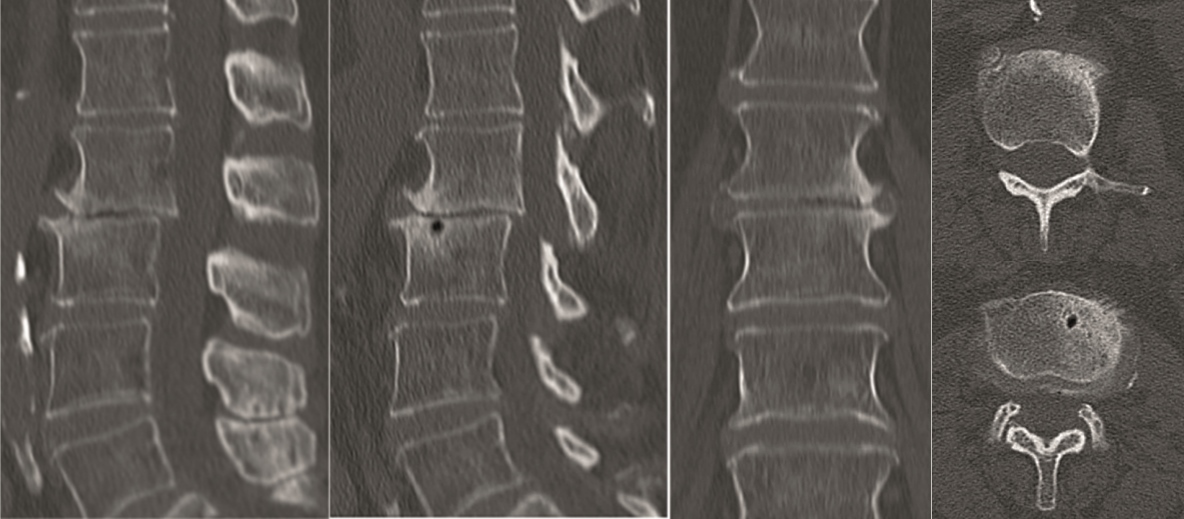
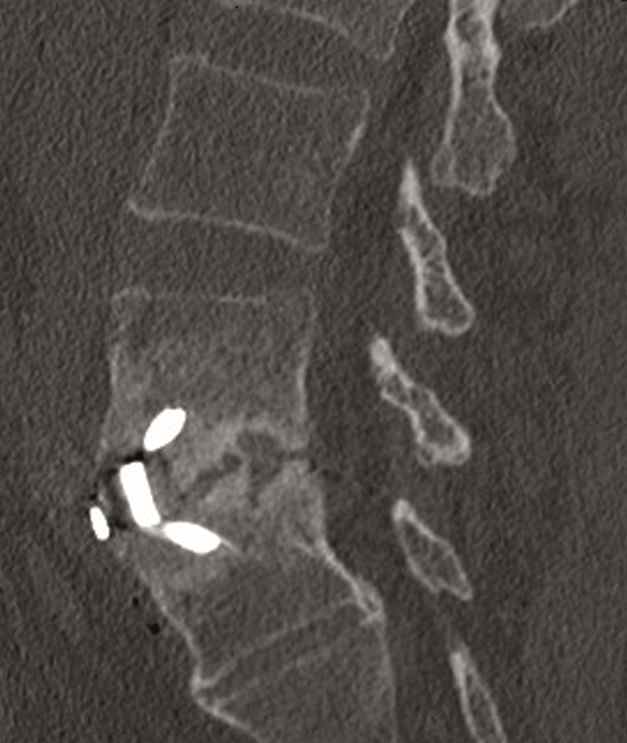
A 40-year-old man 5 years after ALIF L4-L5 using SynFix with axial low back pain. The CT scan shows locked pseudarthrosis (Fig 17). Nonoperative treatment failed. The treatment option was bilateral Facet Wedge at L4-L5.
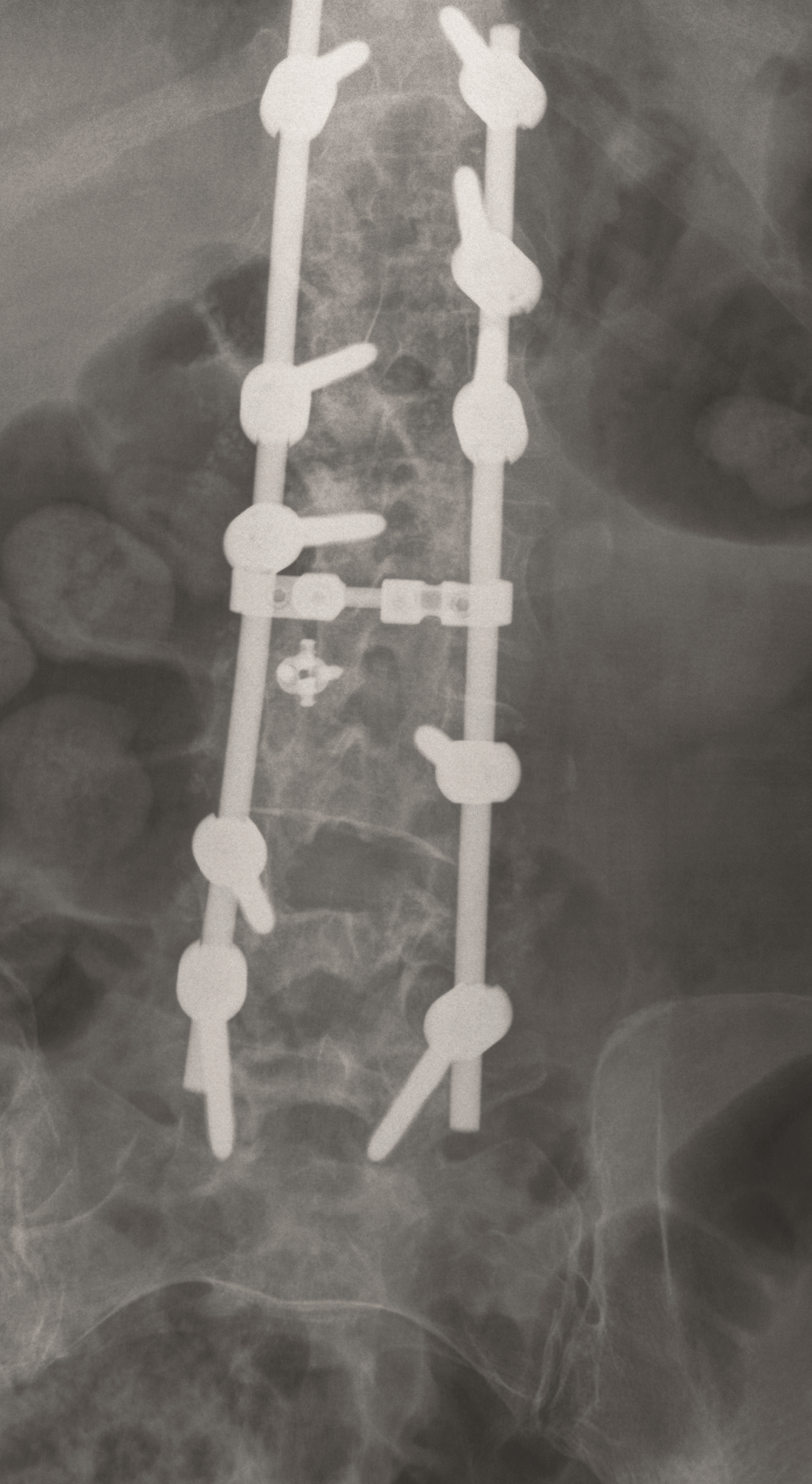
A less invasive approach was used with Insight Retractor using the bilateral Facet Wedge. No bone graft. X-ray follow-up after 3 months and CT assessment after 6 months (Fig 18-19).
Case 5 : Degenerative scoliosis
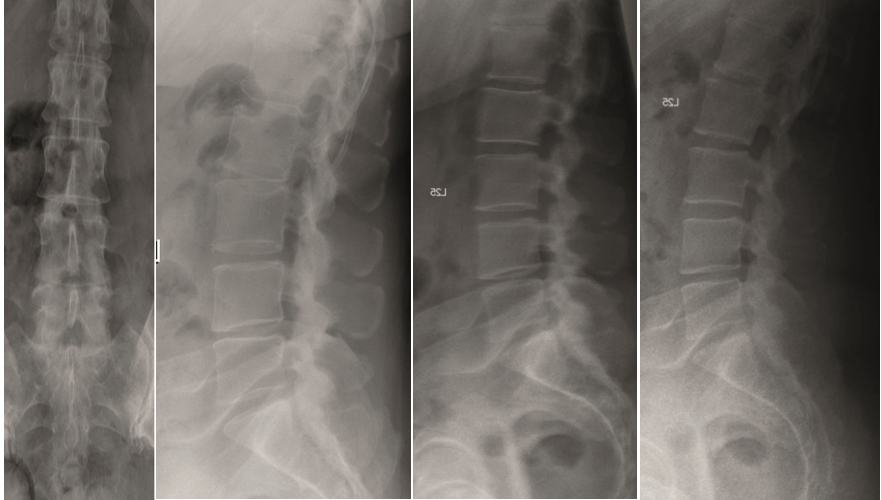
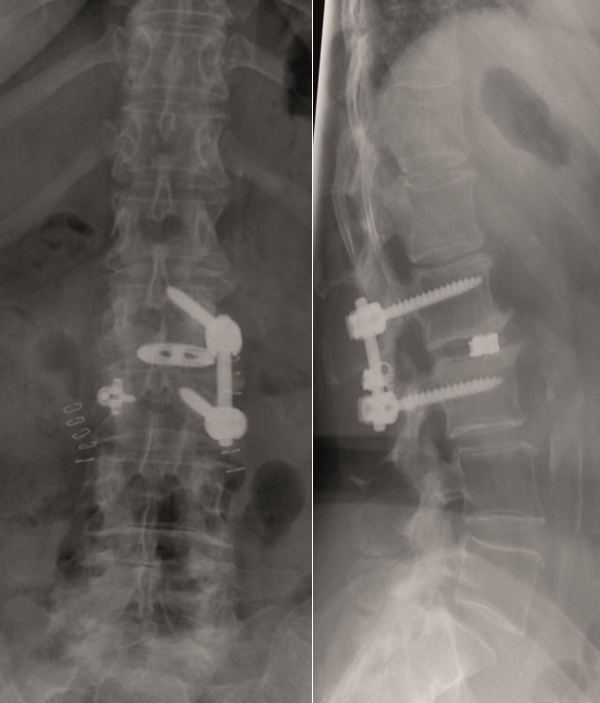
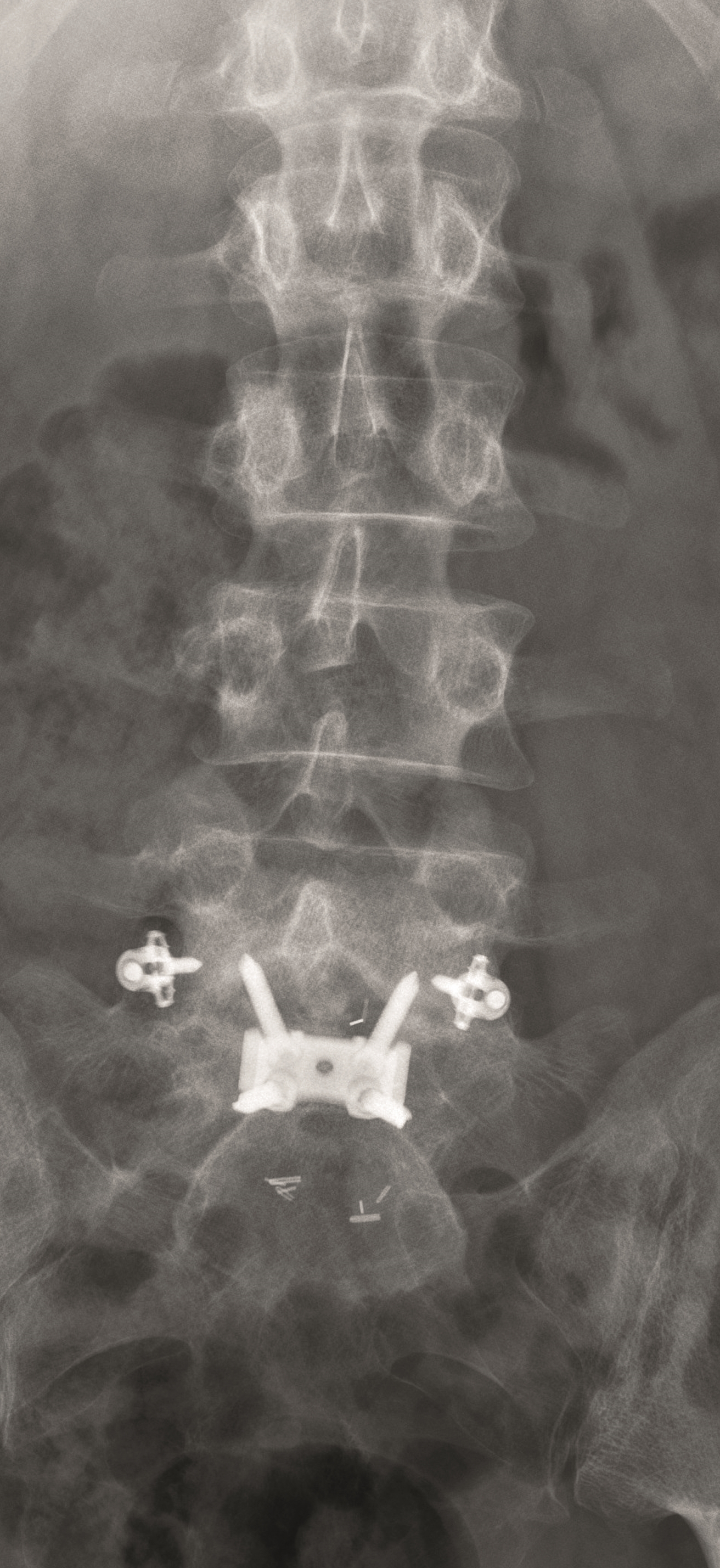
A female patient 66-years-old with back pain, leg pain, and degenerative deformity. The x-rays show left convex degenerative scoliosis Cobb T12-L3 38. Nonoperative treatment failed. Treatment option was posterior fusion T11-L5, with URS Facet Wedge L2-L3 unilaterally.
A conventional approach for posterior correction was taken, with indirect Foraminal decompression and Facet Wedge fusion (apex curve). Facet Wedge introduction after curve correction with rod in situ. X-ray follow-up initially (Fig 20), with CT assessment of Facet Wedge fusion after 6 months (Fig 21).
Primary stability and permanent fusion through locking of the facet joints.
Hazards and labeling
Due to varying countries’ legal and regulatory approval requirements, consult the appropriate local product labeling for approved intended use of the products described on this website. All devices on this website are approved by the AO Technical Commission. For logistical reasons, these devices may not be available in all countries worldwide at the date of publication.
Legal restrictions
This work was produced by AO Foundation, Switzerland. All rights reserved by AO Foundation. This publication, including all parts thereof, is legally protected by copyright.
Any use, exploitation or commercialization outside the narrow limits set forth by copyright legislation and the restrictions on use laid out below, without the publisher‘s consent, is illegal and liable to prosecution. This applies in particular to photostat reproduction, copying, scanning or duplication of any kind, translation, preparation of microfilms, electronic data processing, and storage such as making this publication available on Intranet or Internet.
Some of the products, names, instruments, treatments, logos, designs, etc referred to in this publication are also protected by patents, trademarks or by other intellectual property protection laws (eg, “AO” and the AO logo are subject to trademark applications/registrations) even though specific reference to this fact is not always made in the text. Therefore, the appearance of a name, instrument, etc without designation as proprietary is not to be construed as a representation by the publisher that it is in the public domain.
Restrictions on use: The rightful owner of an authorized copy of this work may use it for educational and research purposes only. Single images or illustrations may be copied for research or educational purposes only. The images or illustrations may not be altered in any way and need to carry the following statement of origin “Copyright by AO Foundation, Switzerland”.
Check www.aofoundation.org/disclaimer for more information.
If you have any comments or questions on the articles or the new devices, please do not hesitate to contact us.
“approved by AO Technical Commission” and “approved by AO”
The brands and labels “approved by AO Technical Commission” and “approved by AO”, particularly "AO" and the AO logo, are AO Foundation's intellectual property and subject to trademark applications and registrations, respectively. The use of these brands and labels is regulated by licensing agreements between AO Foundation and the producers of innovation products obliged to use such labels to declare the products as AO Technical Commission or AO Foundation approved solutions. Any unauthorized or inadequate use of these trademarks may be subject to legal action.
AO ITC Innovations Magazine
Find all issues of the AO ITC Innovations Magazine for download here.
Innovation Awards
Recognizing outstanding achievements in development and fostering excellence in surgical innovation.