ZERO-P ChronOS
Stability is a clear prerequisite for an implant to guarantee optimal healing results. Current state-of-the-art solutions for anterior cervical discectomy and fusion (ACDF) include existing nonfilled zero-profile devices, such as ZERO-P and ZERO-P VA, but also include cervical cages with anterior cervical plate fixation.
So far, biomechanical testing has already shown the stability of ZEROP to be similar to that of traditional cage and plate constructs [1]. Further, clinical studies have demonstrated that ZERO-P implants filled with standard ChronOS cylinders for ACDF show excellent fusion results [2].
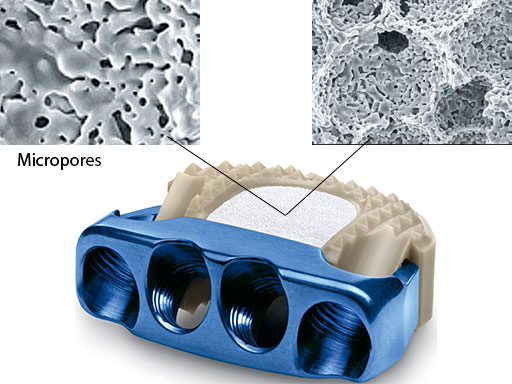
Now, the ZERO-P ChronOS is available. ZERO-P ChronOS (Fig 1) is prefilled with a bioresorbable synthetic beta-tricalcium phosphate cancellous bone substitute (ChronOS). ChronOS is osteoconductive and osteopromotive when perfused with bone marrow.
ZERO-P ChronOS is based on the ZERO-P surgical technique and no additional instruments are needed.
The indications for ZERO-P ChronOS are identical for those for the existing ZERO-P implant:
- Degenerative disc diseases
- Spinal stenosis
- Pseudoarthrosis.
In addition, ZERO-P ChronOS provides the following helpful features:
- Reduced OR time
- Less donor site morbidity
- Increased safety
- Enhanced fusion.
With no extra filling step required, there is reduced OR time as implants can be directly used after unpacking (to ensure rapid onset of fusion of the prefilled ZERO-P and subsequent remodeling of the ChronOS insert). The implant must be augmented with autologous blood or bone marrow aspirate. Soaking in blood should be done for at least 15 seconds to ensure sufficient perfusion of the ChronOS insert (Fig 2).
There is less donor site morbidity as there is no need for secondary surgery to remove autologous bone.
There is increased safety as the device is 100?% synthetic, with no risk of cross infection. The synthetic bioresorbable material converts to vital bone within 6-18 months and is an alternative to allograft.
Finally, the device provides enhanced fusion for patients with expected or already experienced difficulties in fusion due to its osteoconductive features (macropores facilitate bone ingrowth, interconnected micropores allow an optimum supply of nutrients) and osteopromotive features (saturation with the patient's own blood or bone marrow during surgery supports bone integration and ensures rapid ongrowth to the implant) (Fig 1).
For optimal adoption to the patient anatomy, ZERO-P ChronOS is available in two sagittal spacer shapes (convex, lordotic), one standard footprint size, and multiple height options (512 mm in 1 mm increments).
References
- Scholz M, Reyes PM, Schleicher P, et al. A new standalone cervical anterior interbody fusion device: biomechanical comparison with established anterior cervical fixation devices: Spine 34, 2009: 156160.
- Scholz M, Schnake KJ, Pingel A, et al. A New Zero-profile Implant for Standalone Anterior Cervical Interbody Fusion. Clin Orthop Relat Res. 2011 March; 469(3):666673.
Case: Cervical radiculo-myelopathy
(Case provided by Frank Kandziora, Frankfurt am Main, Germany)
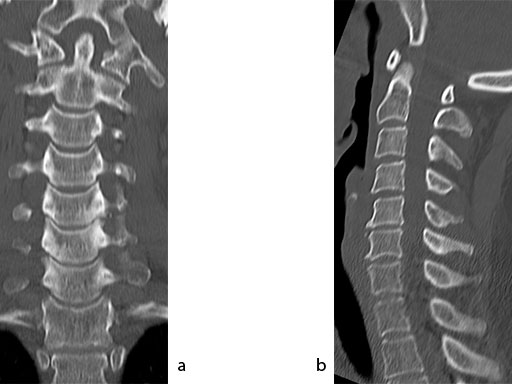
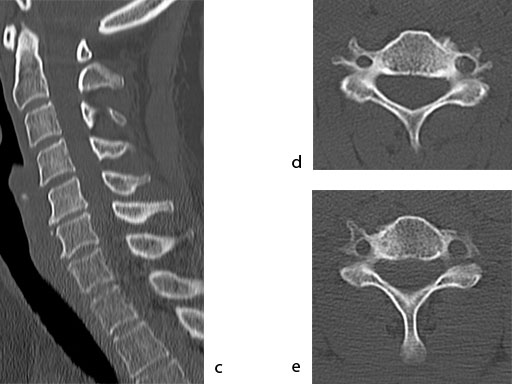
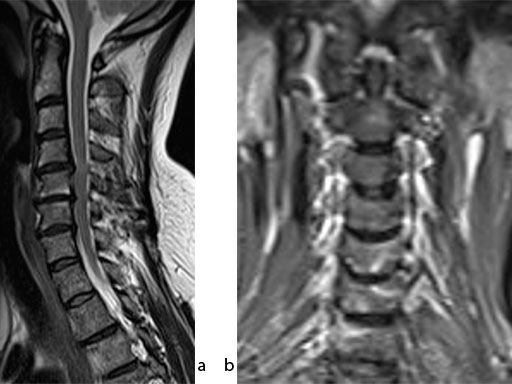
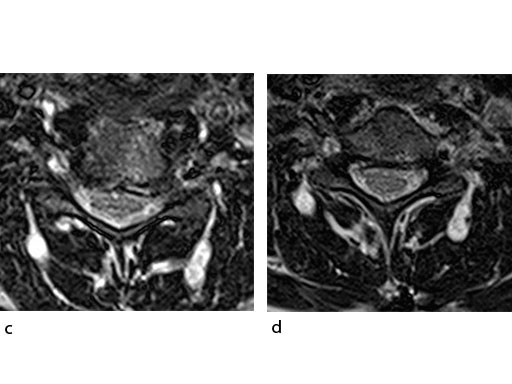
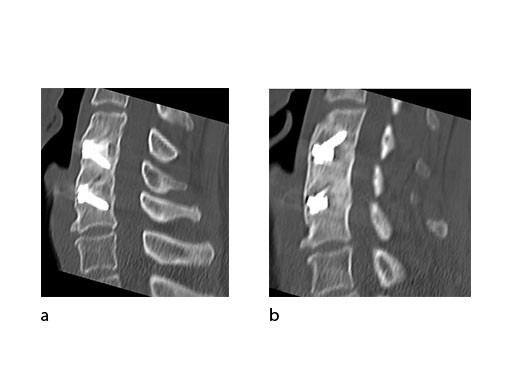
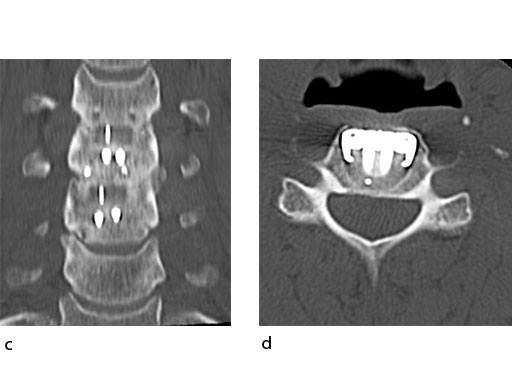
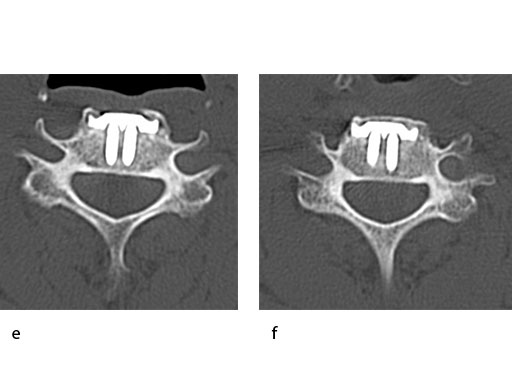
A 51-year-old woman was affected by cervical radiculo-myelopathy due to spinal and neuroforaminal stenosis. The preoperative CT scans are shown at Fig 4 and MRI at Fig 5.
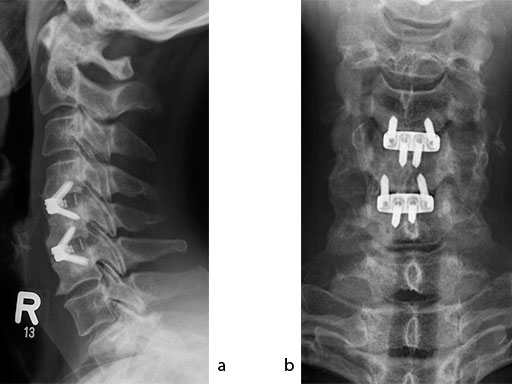
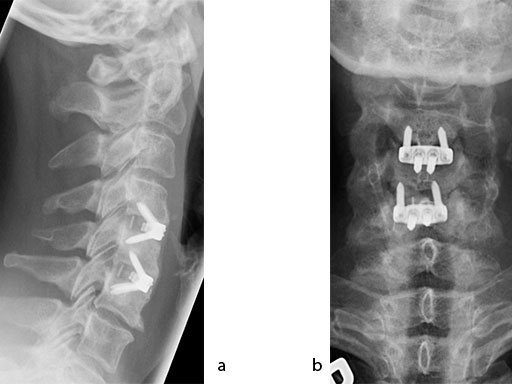
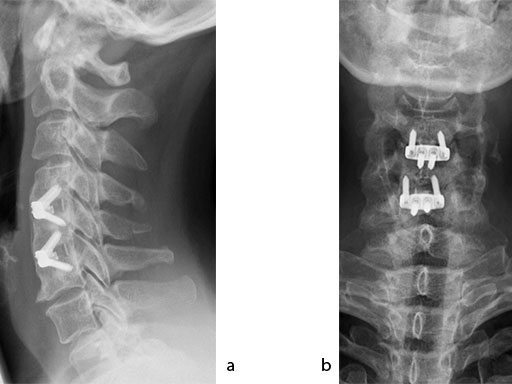
The patient was treated by anterior cervical decompression and fusion (ACDF) with ZERO-P filled with ChronOS. No autologous bone graft was used. Postoperative images are shown (Fig 69).
Enhanced fusion performances in Zero-P through prefilled -tricalcium phosphate
Hazards and labeling
Due to varying countries’ legal and regulatory approval requirements, consult the appropriate local product labeling for approved intended use of the products described on this website. All devices on this website are approved by the AO Technical Commission. For logistical reasons, these devices may not be available in all countries worldwide at the date of publication.
Legal restrictions
This work was produced by AO Foundation, Switzerland. All rights reserved by AO Foundation. This publication, including all parts thereof, is legally protected by copyright.
Any use, exploitation or commercialization outside the narrow limits set forth by copyright legislation and the restrictions on use laid out below, without the publisher‘s consent, is illegal and liable to prosecution. This applies in particular to photostat reproduction, copying, scanning or duplication of any kind, translation, preparation of microfilms, electronic data processing, and storage such as making this publication available on Intranet or Internet.
Some of the products, names, instruments, treatments, logos, designs, etc referred to in this publication are also protected by patents, trademarks or by other intellectual property protection laws (eg, “AO” and the AO logo are subject to trademark applications/registrations) even though specific reference to this fact is not always made in the text. Therefore, the appearance of a name, instrument, etc without designation as proprietary is not to be construed as a representation by the publisher that it is in the public domain.
Restrictions on use: The rightful owner of an authorized copy of this work may use it for educational and research purposes only. Single images or illustrations may be copied for research or educational purposes only. The images or illustrations may not be altered in any way and need to carry the following statement of origin “Copyright by AO Foundation, Switzerland”.
Check www.aofoundation.org/disclaimer for more information.
If you have any comments or questions on the articles or the new devices, please do not hesitate to contact us.
“approved by AO Technical Commission” and “approved by AO”
The brands and labels “approved by AO Technical Commission” and “approved by AO”, particularly "AO" and the AO logo, are AO Foundation's intellectual property and subject to trademark applications and registrations, respectively. The use of these brands and labels is regulated by licensing agreements between AO Foundation and the producers of innovation products obliged to use such labels to declare the products as AO Technical Commission or AO Foundation approved solutions. Any unauthorized or inadequate use of these trademarks may be subject to legal action.
AO ITC Innovations Magazine
Find all issues of the AO ITC Innovations Magazine for download here.
Innovation Awards
Recognizing outstanding achievements in development and fostering excellence in surgical innovation.