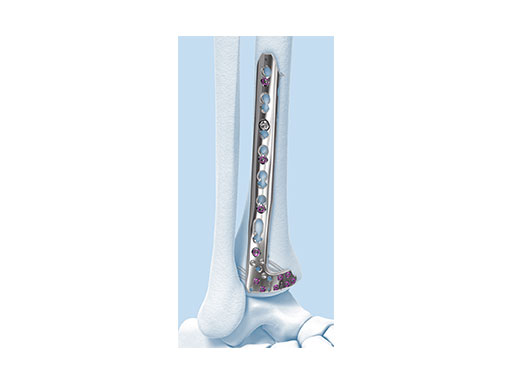
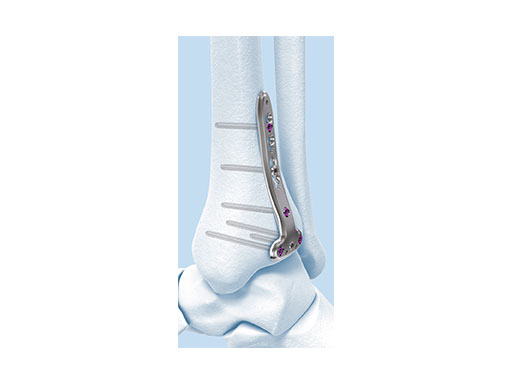
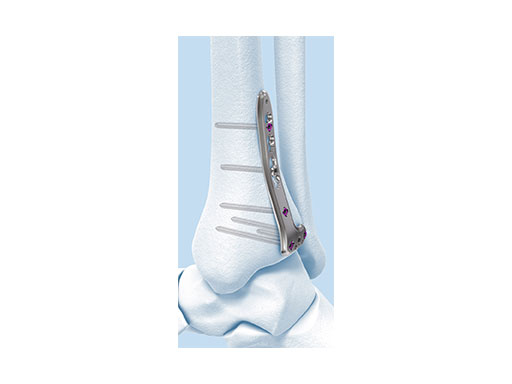
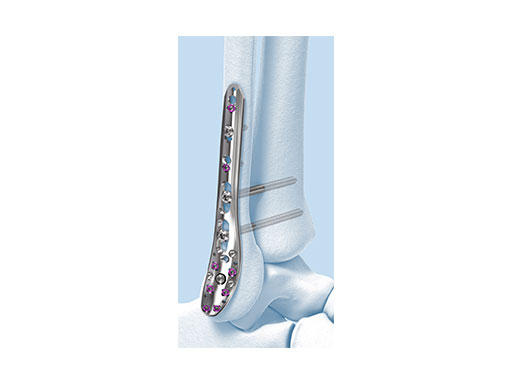
Variable Angle LCP Ankle Trauma 2.7/3.5 System
Paul Barbosa, Mark Lee
Treatment of complex ankle fractures when combined with poor bone quality can be challenging. A considerable rate of unsatisfactory results has been observed due to a loss of reduction, nonunions, and impaired function when using current plating systems. These plating options are sometimes also considered as being too thick, and may irritate soft tissues.
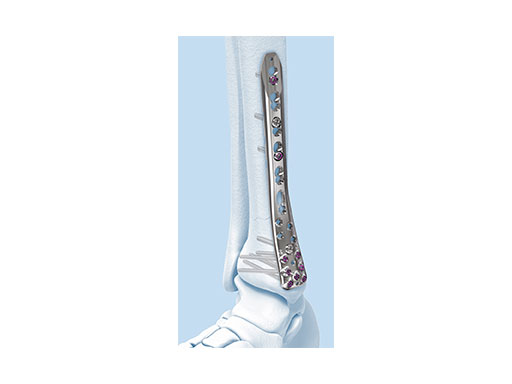
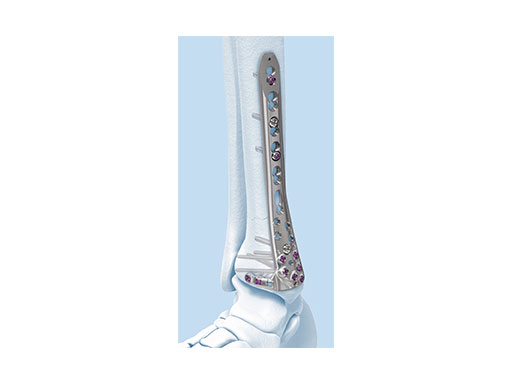
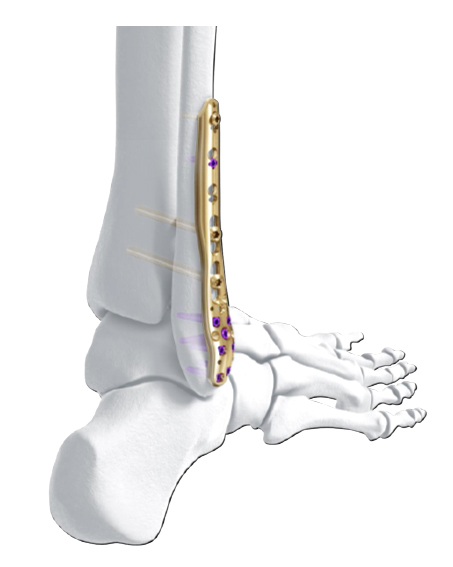
The new Variable Angle LCP Ankle Trauma 2.7/3.5 system was developed to overcome these shortcomings. The low-profile plates feature variable angle locking screw technology and offer more options for screw placement to address a wider range of fracture patterns and to accommodate varied patient anatomies. In addition, these anatomically accurate plate designs simplify minimal invasive plate insertion and indirect reduction techniques (push-pull applications). The plates are available in stainless steel and are primarily for the treatment of articular injuries. The VA-LCP Lateral Distal Fibula Plates are also provided in titanium.
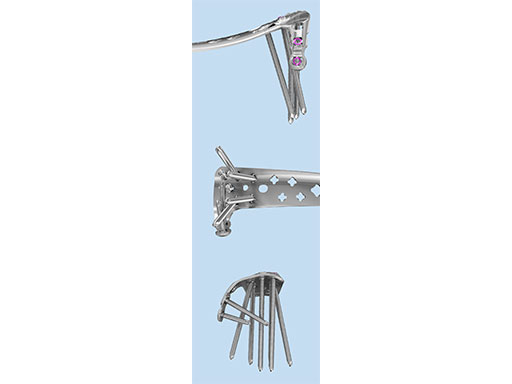
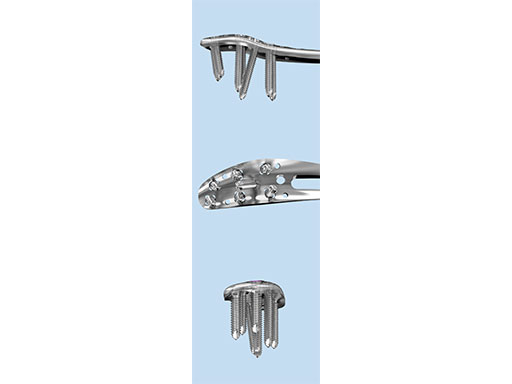
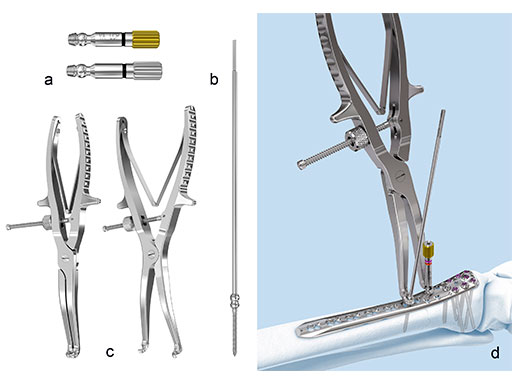
The Combi holes in the Variable Angle LCP plate shaft combine a dynamic compression unit hole with a variable angle locking screw hole. Due to the smaller sized and more numerous fixation options distally with 2.7 mm VA screws, the fixed-angle construct provides advantages for small metaphyseal segments where traditional screw fixation can be limited. The screw trajectories are optimized to support the distal articular surface. The multiple variable angle locking holes in the plate head accept 2.7 mm VA locking, 2.7 mm locking, 2.7 mm cortex, and 2.7 mm metaphyseal screws. The latter has a low profile screw head to avoid soft tissue irritations and can be used to pull the plate to the bone. Due to the numerous screw possibilities, it is important that the surgeon is familiar with which screws can be used in which plate holes. The system includes guide blocks for all plates (except T and L plates) for inserting screws in the plate head at nominal screw angles (Fig 1a-c). K-wire holes in the plate head and shaft tip accept K-wires up to 1.6 mm. They can be used to temporarily reduce articular fragments, and to confirm the location of the plate relative to the distal tibia and fibula.
Design features
The design features of some of the plates are as follows:
- VA-LCP Anteromedial Distal Tibia Plate: The anterior arm of the plate aids in capturing small articular bone fragments and has three 2.7 mm VA locking screw holes
- VA-LCP Anterolateral Distal Tibia Plate: The plate is designed to be placed more distally than the previous 3.5 mm LCP Anterolateral Distal Tibia Plate. It also provides more coverage in the distal anterolateral area. Screw angulations at nominal angle are targeted for Volkmann's triangle and the Chaput fragments
- VA-LCP Lateral Distal Fibula Plate: The plate contour is designed to provide plate contact at the distal tip and on the proximal end, which allows the plate to avoid 'rocking' on the fibular ridge. In cadaveric evaluations, the fibular ridge was found to be highly variable in both height and length. The under-contour bypasses the variability and assists in reduction by minimizing a valgus deformity if the plate is in contact with the ridge. The plate offers two syndesmotic slots that accept 3.5 mm low profile cortex or 4.0 mm cortex screws.
The design rationale for the Variable Angle LCP Ankle Trauma 2.7/3.5 system has paralleled the evolution of approaches to periarticular fracture care. With improved appreciation of the benefits of fracture configuration- guided surgical exposures, a greater variation of plating surfaces are now utilized. New plate contours are essential to optimize buttressing function and distal screw positions. The new complement of ankle plates provides ideally contoured plates for all common plate positions around the tibia and fibula. The new plate shape design has improved the distal fit of all of the plates, and minimal contour is necessary to obtain full surface plate bone apposition. The posterior tibial plate options provide excellent posterior buttress for posterior partial articular fragments, even via the posterolateral insertion portal.
One of the greatest advantages of the new plates is the VA feature that allows for targeting of specific fracture fragments. Additionally, the ability to self-direct these screw paths optimizes the ability to use these plates with other implants (both plates and intramedullary devices) and essentially solves the problem of screw interference/traffic from supplementary plate positions, which are commonly utilized for high energy fracture variants. These plates should be applied using traditional techniques. Additionally, while the VA option can be utilized, the nominal screw positions provide excellent screw distribution, good articular support, and optimal angular stability. The VA option should be reserved for situations where alternative screw positions are critical, especially in situations where optimal angular stability is desired.
An essential benefit of the plating system is the option to have a fully self-contained system with a full complement of plates, screw types, and instrumentation; there is almost no need for supplemental hardware sets to be opened. The sets of the Variable Angle LCP Ankle Trauma system are modular to allow customized selection of implants, which also reduces inventory and overall costs by eliminating seldom-used implants.
The Variable Angle LCP Ankle Trauma system also comprises a compression and distraction system, which is an important tool for reduction (i.e. bringing a fibula out to length) or compression of a fracture through the plate itself (Fig 9a-d). The forceps allow for final screw fixation after compression or distraction has been achieved.
Case 1: Snowboarding accident (Case provided by Mark Lee, Sacramento, USA)
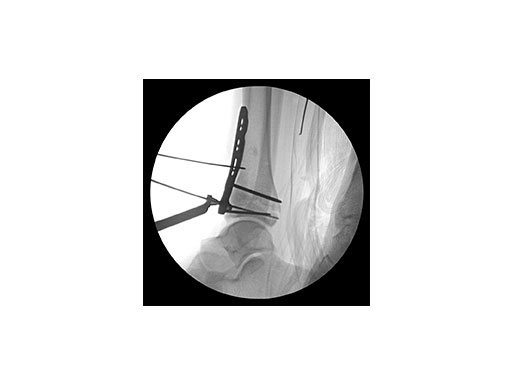
A 17-year-old male patient was involved in a snowboarding accident. This was a closed injury, and he was initially splinted for 10 days. His injury pattern demonstrated anterior articular impaction (Fig 1).
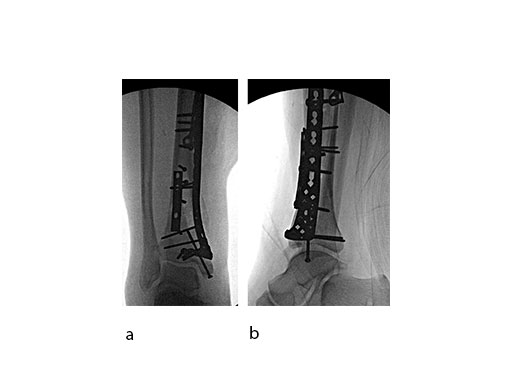
Surgeons performed an anterolateral surgical approach and distracted the joint using the distraction clamp and osteotome (Fig 2). The fracture was fixated with a VA-LCP Anterolateral Distal Tibia Plate (Fig 35).
Case 2: Scaffolding fall (Case provided by Mark Lee, Sacramento, USA)
A 61-year-old laborer fell 5 meters from scaffolding. This was a closed injury with severe soft-tissue injury (Fig 67). He required three weeks of jointspanning external fixation prior to adequate resolution of edema.
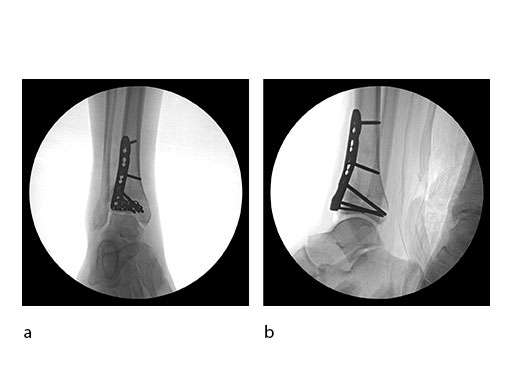
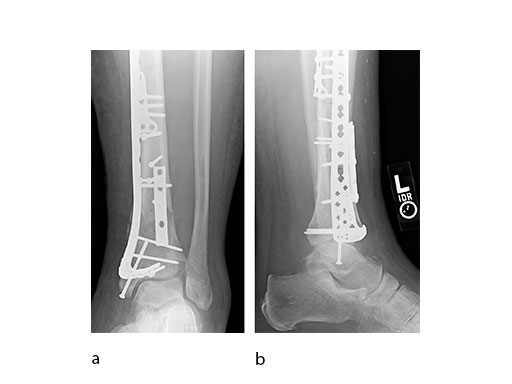
Initial fixation was via a limited posteromedial exposure to buttress a posteromedial partial articular fragment (Fig 8). Following articular reduction and supplemental lateral column plate fixation via a limited anteromedial joint exposure, the VA-LCP Anteromedial Distal Tibia Plate was passed subcutaneous with subsequent percutaneous screw insertions into the plate shaft.
There was good maintenance of reduction at 5 weeks (Fig 9).
Case 3: Horse fall (Case provided by Mark Lee, Sacramento, USA)
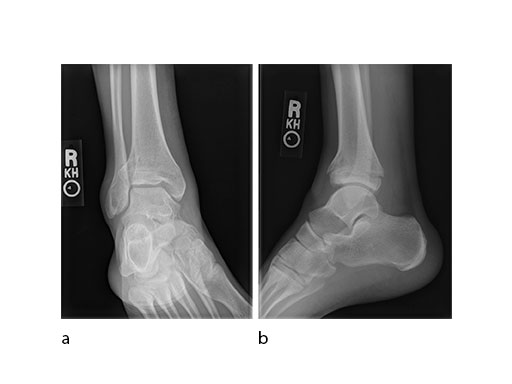
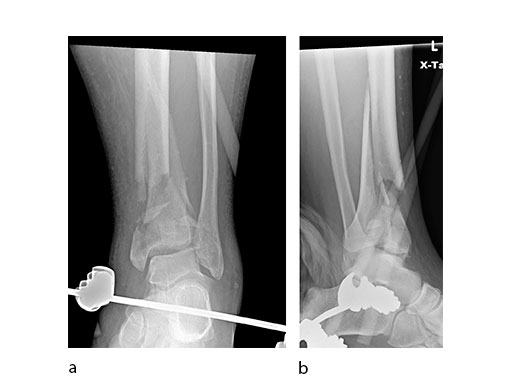
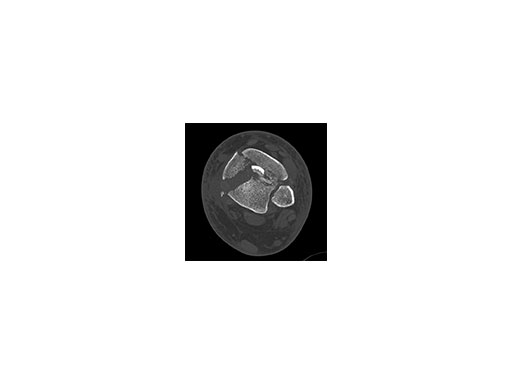
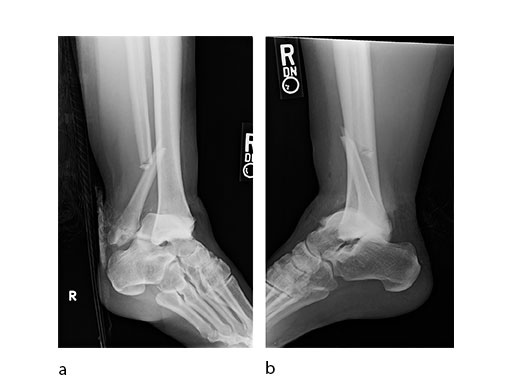
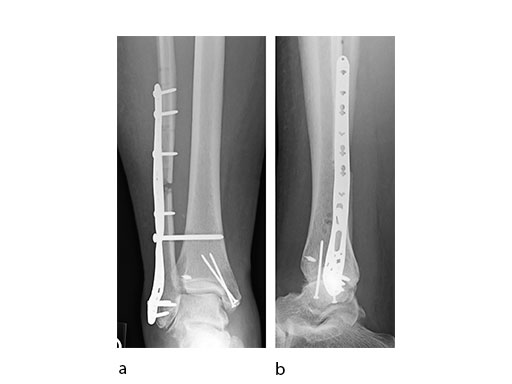
A 45-year-old patient fell from his horse, receiving a closed bimalleolar fracture/ dislocation (Fig 10). Computed tomorgraphy demonstrated an additional anterolateral avulsion injury and syndesmosis dislocation. Osteosynthesis was conducted with a VA-LCP Lateral Distal Fibula Plate.
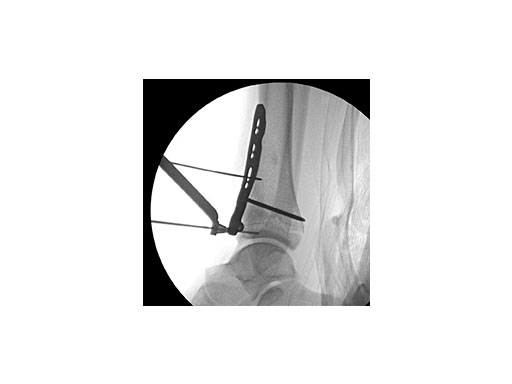
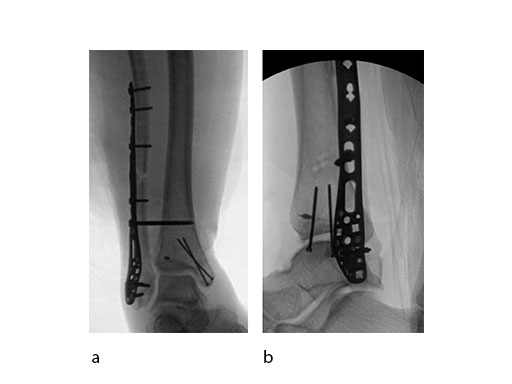
Push technique was required to achieve fibular reduction, and the syndesmosis was reduced and clamped with periarticular clamps (Fig 11). Postoperative x-rays at 4 weeks demonstrated good maintenance of reduction (Fig 12).
Options for locking compression in distal tibia and fibula fractures
Hazards and labeling
Due to varying countries’ legal and regulatory approval requirements, consult the appropriate local product labeling for approved intended use of the products described on this website. All devices on this website are approved by the AO Technical Commission. For logistical reasons, these devices may not be available in all countries worldwide at the date of publication.
Legal restrictions
This work was produced by AO Foundation, Switzerland. All rights reserved by AO Foundation. This publication, including all parts thereof, is legally protected by copyright.
Any use, exploitation or commercialization outside the narrow limits set forth by copyright legislation and the restrictions on use laid out below, without the publisher‘s consent, is illegal and liable to prosecution. This applies in particular to photostat reproduction, copying, scanning or duplication of any kind, translation, preparation of microfilms, electronic data processing, and storage such as making this publication available on Intranet or Internet.
Some of the products, names, instruments, treatments, logos, designs, etc referred to in this publication are also protected by patents, trademarks or by other intellectual property protection laws (eg, “AO” and the AO logo are subject to trademark applications/registrations) even though specific reference to this fact is not always made in the text. Therefore, the appearance of a name, instrument, etc without designation as proprietary is not to be construed as a representation by the publisher that it is in the public domain.
Restrictions on use: The rightful owner of an authorized copy of this work may use it for educational and research purposes only. Single images or illustrations may be copied for research or educational purposes only. The images or illustrations may not be altered in any way and need to carry the following statement of origin “Copyright by AO Foundation, Switzerland”.
Check www.aofoundation.org/disclaimer for more information.
If you have any comments or questions on the articles or the new devices, please do not hesitate to contact us.
“approved by AO Technical Commission” and “approved by AO”
The brands and labels “approved by AO Technical Commission” and “approved by AO”, particularly "AO" and the AO logo, are AO Foundation's intellectual property and subject to trademark applications and registrations, respectively. The use of these brands and labels is regulated by licensing agreements between AO Foundation and the producers of innovation products obliged to use such labels to declare the products as AO Technical Commission or AO Foundation approved solutions. Any unauthorized or inadequate use of these trademarks may be subject to legal action.
AO ITC Innovations Magazine
Find all issues of the AO ITC Innovations Magazine for download here.
Innovation Awards
Recognizing outstanding achievements in development and fostering excellence in surgical innovation.