LCP Ulna Osteotomy 2.7 System
Ulna impaction syndrome (or ulnocarpal abutment syndrome) is a degenerative condition related to excessive load bearing across the ulna aspect of the wrist, which results in chronic impingement of the ulna head against TFCC, lunate and triquetrum. This chronic impingement can cause wrist pain, swelling, limited range of motion and diminished grip strength. In most cases, a positive ulna variance causes ulnocarpal impaction syndrome. Distal radius fractures with radius collapse are also a common problem with a secondary painful positive ulna variance. Depending on stage and patient symptomatology, the treatment includes an ulna shortening osteotomy although common complications can include hardware irritations and delayed or non-unions.
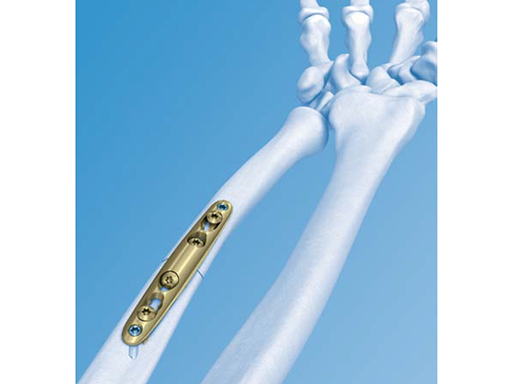
The LCP Ulna Osteotomy 2.7 system (Fig 1a) is ideal for shortening osteotomies of the ulna. It allows accurate oblique or transverse osteotomy cuts and correct restoration of bone alignment, and uses a smooth and low profile plate design that minimizes hardware irritation. The system consists of plates in two sizes - short plate 6-hole, and long plate 8-hole. (Fig 1b)
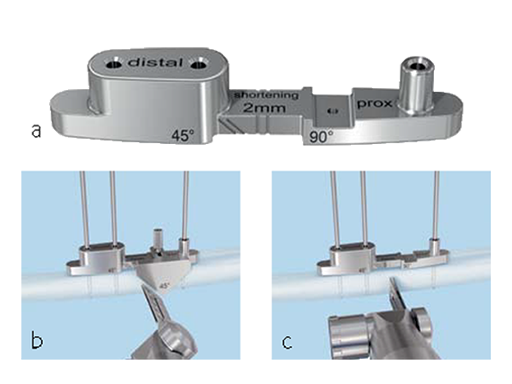
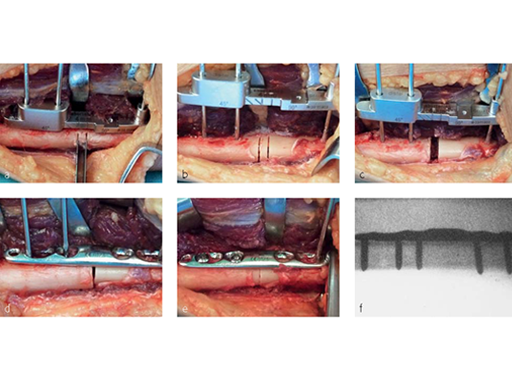
The system consists of five drill templates with predefined shortening lengths (2.0 mm/2.5 mm/3.0 mm/4.0 mm/5.0 mm) for transverse or oblique cuts. (Fig 3) In addition, the system contains a saw guide for oblique 45 cuts, a 2.0 mm K-wire with drill tip, five parallel saw blades for transverse cuts, and five parallel saw blades for oblique (45) cuts (Fig 3)
Technique for usage
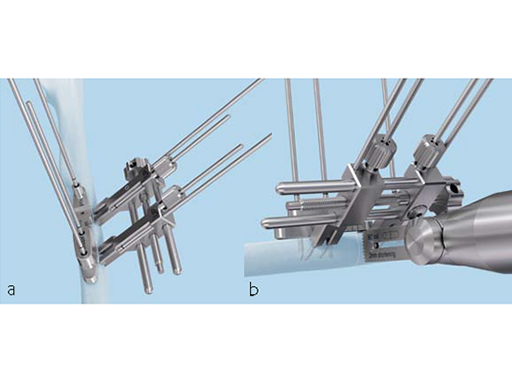
First of all, either a transverse or oblique osteotomy has to be selected. Screw holes for the plate need to be drilled at the right place before the osteotomy is done. A special tool helps get a precise parallel osteotomy cut, but the instrument will only work on a flat surface. A bent or curved bone will require a larger incision. If the plate toggles, either the bone needs to be flattened or the plate should be positioned more proximally. For an oblique cut, a guide is used for marking the osteotomy and then removed. The correct usage of the jig (Fig 4) is important for accurate shortening, and proper rotation alignment needs to be ensured. Sufficient inter-fragmentary compression (good friction of the whole osteotomy surface) is needed. In oblique osteotomies, the adequate length of the lag screw (screws should be used 1 mm longer than measured) is mandatory for a good compression. Depending on bone quality and the amount of shortening required, a sufficient number of bi-cortical locking screws has to be used. In patients with hard bone, it is advisable to use the dedicated tap.
In summary, the LCP Ulna Osteotomy 2.7 system leads to a lower complication rate, reduced non-union rate, and reduced postoperative pain, as it allows for a shorter incision, more precise cutting, better alignment, and minimizes hardware irritation, which greatly reduces the need for plate removal, if the correct surgical technique is followed.
Case 1: Distal radius fracture
Cases provided by Doug Campbell, Leeds, UK; Ladislav Nagy, Zurich, Switzerland; and Juan Gonzlez del Pino, Madrid, Spain.
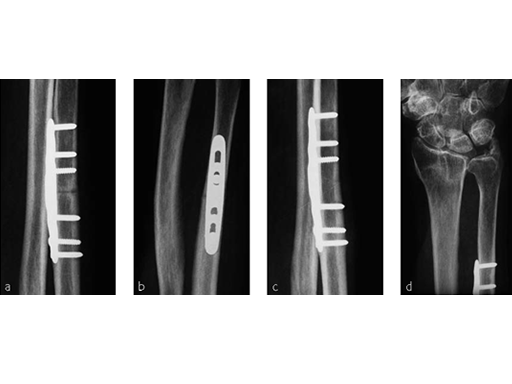
A 69-year-old female patient had suffered a right distal radius fracture one year earlier, and received conservative management. Symptoms included pain and impaired function about the wrist and forearm, with decreased forearm rotation. Painful DRUJ (DASH: 34, PWRE: 29).
Case 2: Painful ulno-carpal abutment
A 32-year-old man suffered torsional trauma about the right wrist, with TCFF rupture. A failed arthroscopic repair had taken place. Constitutional ulna plus. Symptoms included pain and impaired function about the wrist and forearm. Painful DRUJ (DASH: 22, PWRE: 21).
Case 3: Oblique osteotomy
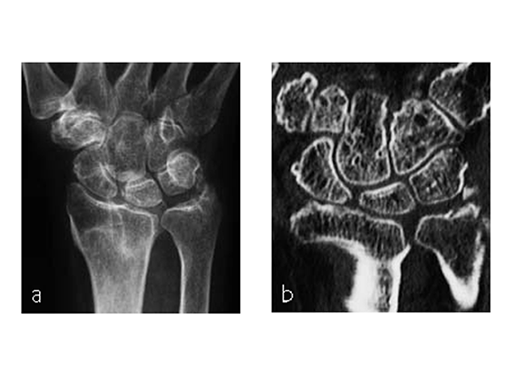
A 48-year-old female nurse had a diagnosis of a degenerative central TFCCrupture, with chronic ulnocarpal abutment.
The amount of correction required was 2.5 mm. The preoperative x-ray showed positive ulna variance.
Hazards and labeling
Due to varying countries’ legal and regulatory approval requirements, consult the appropriate local product labeling for approved intended use of the products described on this website. All devices on this website are approved by the AO Technical Commission. For logistical reasons, these devices may not be available in all countries worldwide at the date of publication.
Legal restrictions
This work was produced by AO Foundation, Switzerland. All rights reserved by AO Foundation. This publication, including all parts thereof, is legally protected by copyright.
Any use, exploitation or commercialization outside the narrow limits set forth by copyright legislation and the restrictions on use laid out below, without the publisher‘s consent, is illegal and liable to prosecution. This applies in particular to photostat reproduction, copying, scanning or duplication of any kind, translation, preparation of microfilms, electronic data processing, and storage such as making this publication available on Intranet or Internet.
Some of the products, names, instruments, treatments, logos, designs, etc referred to in this publication are also protected by patents, trademarks or by other intellectual property protection laws (eg, “AO” and the AO logo are subject to trademark applications/registrations) even though specific reference to this fact is not always made in the text. Therefore, the appearance of a name, instrument, etc without designation as proprietary is not to be construed as a representation by the publisher that it is in the public domain.
Restrictions on use: The rightful owner of an authorized copy of this work may use it for educational and research purposes only. Single images or illustrations may be copied for research or educational purposes only. The images or illustrations may not be altered in any way and need to carry the following statement of origin “Copyright by AO Foundation, Switzerland”.
Check www.aofoundation.org/disclaimer for more information.
If you have any comments or questions on the articles or the new devices, please do not hesitate to contact us.
“approved by AO Technical Commission” and “approved by AO”
The brands and labels “approved by AO Technical Commission” and “approved by AO”, particularly "AO" and the AO logo, are AO Foundation's intellectual property and subject to trademark applications and registrations, respectively. The use of these brands and labels is regulated by licensing agreements between AO Foundation and the producers of innovation products obliged to use such labels to declare the products as AO Technical Commission or AO Foundation approved solutions. Any unauthorized or inadequate use of these trademarks may be subject to legal action.
AO ITC Innovations Magazine
Find all issues of the AO ITC Innovations Magazine for download here.
Innovation Awards
Recognizing outstanding achievements in development and fostering excellence in surgical innovation.