Zero-P VA
Cervical Degenerative Disc Disease (DDD) is a common pathology and part of the natural process of aging. When DDD becomes symptomatic or painful, it can cause several different symptoms, including neck pain, nerve root pathology (eg, numbness or pain in shoulders/arms), and spinal cord compression. If pain cannot be resolved by conservative treatment, surgery might become the only option. Some patients with persistent pain or neurological deficits need surgery to relieve the symptoms.
Todays standard surgical treatment is to fuse the vertebrae adjacent to the diseased disc. Studies show that anterior cervical plating helps increase fusions rates [1,2], however anterior cervical plating also has potential drawbacks, such as the prolongation of OR time, the potential for postoperative dysphagia, and/or heterotopic ossification (abnormal formation of bone over time) at adjacent spinal levels.
In 2008, a novel cervical fusion implant named Zero-P was introduced. The implant was designed to be fully contained within the excised disc space without protruding past the anterior wall of the vertebral body when implanted. The combination of the plate/spacer assembly with four rigid screws ensures that the construct provides similar stability [3] to traditional cervical plate and spacer constructs while offering a number of clinical advantages. These include:
- Prevention of soft-tissue irritation
- Minimization of adjacent level ossification
- Smaller incision sizes
- Facilitates surgeries where Zero-P is implanted adjacent to a previous fusion.
Zero-P VA (Fig.1) complements the original Zero-P (Fig.2) by offering an implant that is based on the same fundamentals but utilizes two semi-constrained screws with variable angle insertion instead of four rigid screws as a means of fixation.
Zero-P VA is designed for surgeons ease of use. It is a stand-alone plate/spacer fusion device for indications where the stability of a two screw semi-constrained fixation is sufficient.
For optimal adoption to the patient anatomy, Zero-P VA is available in various spacer shapes (convex, lordotic, parallel), two different footprint sizes and multiple height options (512 mm in 1 mm increments).
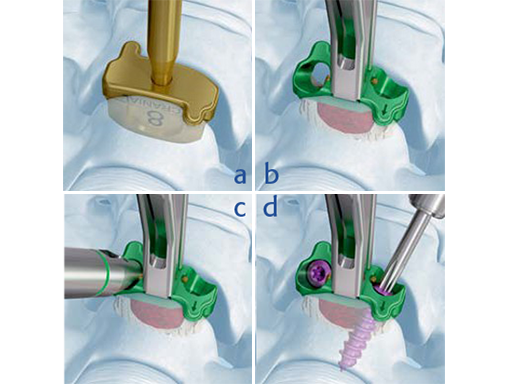
Zero-P VA follows a similar surgical technique to Zero-P. In a first step after discectomy and decompression, trial spacers are used to determine implant height, shape, and footprint size. Once the implant is inserted and correctly positioned, screw hole preparation is performed. For anatomically challenging situations, such as patients with short necks, angled instruments are available. Blocking of the screw happens automatically once the screw head passes the golden blocking pin built into the plate. This one-step blocking mechanism features audible, tactile and visual cues to confirm the screw is blocked upon insertion and ensures its secure retention.
References
- Kaiser MG, Haid RW Jr, Subach BR, et al (2002) Anterior cervical plating enhances arthrodesis after discectomy and fusion with cortical allograft. Neurosurgery; 50(2):229236.
- Fraser JF, Hrtl R (2007) Anterior approaches to fusion of the cervical spine: a metaanalysis of fusion rates. J Neurosurgery Spine; Spine 6(4):298303.
- Scholz M, Reyes PM, Schleicher P, et al (2009) A new standalone cervical anterior interbody fusion device: Biomechanical comparison with established anterior cervical fixation devices. Spine 34(2):156160.
- Scholz M, Schnake KJ, Pingel A, et al(2011) A new zero-profile implant for stand-alone anterior cervical interbody fusion. Clin Orthop Relat Res 469(3):666673.
- Vanek P, Bradc O, Saur K (2011) [Anterior interbody fusion of the cervical spine with a zero-p spacer: radiographic results with a minimum follow-up of one year in a prospective study]. Acta Chir Orthop Traumatol Cech 78(6):562567 (Czech).
- Azab W, Abdel-Razek M, Ali A, et al (2012) Outcome evaluation of a zero-profile implant for anterior cervical diskectomy with fusion. Turkish Neurosurgery 22(5):611617.
Zero-P VA main technique steps: a) Trialing 617, b) Spacer insertion, c) Screw hole preparation, d) Screw insertion
Case: Cervicobrachial Pain Syndrome
Case provided by Raoul Heilbronner, St. Gallen, Switzerland
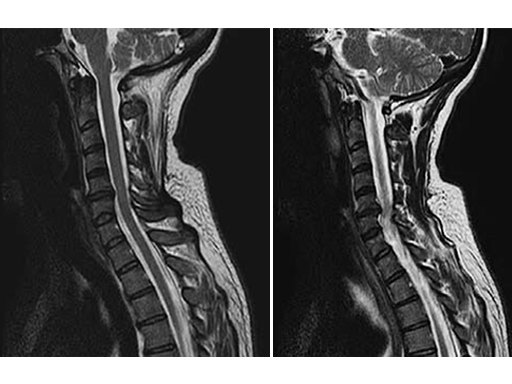
A 49-year-old woman complained of a persistent cervicobrachial pain syndrome on the right side, over a period of five years, which was refractory to all conservative treatments. The conventional x-ray of the cervical spine showed a slightly pronounced degeneration with kyphosis of the segment C5/6, and preoperative MRI showed disc protusion (fig.1).
After an uneventful surgical procedure (Fig. 2) and recovery, the preoperative pain syndrome resolved completely.
Hazards and labeling
Due to varying countries’ legal and regulatory approval requirements, consult the appropriate local product labeling for approved intended use of the products described on this website. All devices on this website are approved by the AO Technical Commission. For logistical reasons, these devices may not be available in all countries worldwide at the date of publication.
Legal restrictions
This work was produced by AO Foundation, Switzerland. All rights reserved by AO Foundation. This publication, including all parts thereof, is legally protected by copyright.
Any use, exploitation or commercialization outside the narrow limits set forth by copyright legislation and the restrictions on use laid out below, without the publisher‘s consent, is illegal and liable to prosecution. This applies in particular to photostat reproduction, copying, scanning or duplication of any kind, translation, preparation of microfilms, electronic data processing, and storage such as making this publication available on Intranet or Internet.
Some of the products, names, instruments, treatments, logos, designs, etc referred to in this publication are also protected by patents, trademarks or by other intellectual property protection laws (eg, “AO” and the AO logo are subject to trademark applications/registrations) even though specific reference to this fact is not always made in the text. Therefore, the appearance of a name, instrument, etc without designation as proprietary is not to be construed as a representation by the publisher that it is in the public domain.
Restrictions on use: The rightful owner of an authorized copy of this work may use it for educational and research purposes only. Single images or illustrations may be copied for research or educational purposes only. The images or illustrations may not be altered in any way and need to carry the following statement of origin “Copyright by AO Foundation, Switzerland”.
Check www.aofoundation.org/disclaimer for more information.
If you have any comments or questions on the articles or the new devices, please do not hesitate to contact us.
“approved by AO Technical Commission” and “approved by AO”
The brands and labels “approved by AO Technical Commission” and “approved by AO”, particularly "AO" and the AO logo, are AO Foundation's intellectual property and subject to trademark applications and registrations, respectively. The use of these brands and labels is regulated by licensing agreements between AO Foundation and the producers of innovation products obliged to use such labels to declare the products as AO Technical Commission or AO Foundation approved solutions. Any unauthorized or inadequate use of these trademarks may be subject to legal action.
AO ITC Innovations Magazine
Find all issues of the AO ITC Innovations Magazine for download here.
Innovation Awards
Recognizing outstanding achievements in development and fostering excellence in surgical innovation.