Zero P
Cervical degenerative disc disease (DDD) is a common pathology and part of the natural process of ageing. When DDD becomes symptomatic or painful, it can cause several different symptoms, including neck pain, nerve root pathology (eg, numbness or pain in shoulders/arms), and spinal cord compression. Some of the patients with persistent pain or neurological deficits need surgery to relieve the symptoms.
So far the standard surgical treatment is to fuse the adjacent vertebrae to the degenerated disc. This is achieved by removing the disc and filling the cavity with an autograft (the patients own bone), allograft (bone from a donor patient) or with a cervical interbody spacer made of titanium or PEEK. Finally the entire segment is fixed with a plate-and-screw construct. In this way the affected segment is fused and stabilized.
Studies show that anterior cervical plating helps increase fusion rates [1, 2], however, anterior cervical plating also has potential drawbacks such as longer surgical times, the potential of postoperative dysphagia and heterotopic ossification at adjacent spinal levels.
A new option for anterior cervical fusion
Zero-P acts as stand-alone implant for use in cervical interbody fusions. Its design combines the functionality of a cervical interbody spacer and the benefits of an anterior cervical plate.
The name Zero-P refers to the zero anterior profile or the fact that it is contained in the excised disc space and does not protrude past the anterior wall of the vertebral body as do anterior cervical plates. Since the implant has zero profile, it may reduce the occurrence of adjacent level ossification and postoperative dysphagia.
Implants and instrumentation
For optimal adoption to the patients anatomy, Zero-P is available in three different spacer shapes (convex, lordodic, and parallel), two different footprint sizes (standard and large) and eight different heights (512 mm). Zero-P is delivered preassembled and sterilely packaged.
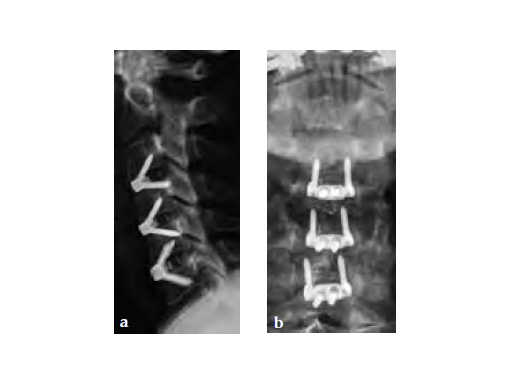
After the removal of the cervical disc, trial spacers are used to determine implant height, shape, and footprint size. After the correct trial spacer is fitted, the corresponding Zero-P implant is inserted using the aiming device. Because plate and spacer are preassembled, the plate is automatically aligned upon implant insertion. This avoids the process of aligning and realigning an anterior cervical plate.
To prepare the screw holes, instruments for awling and drilling are available. For anatomically challenging situations like patients with short necks, angled instruments are available. The Zero-P screws have a one-step locking conical head, which locks the screw to the plate by simply inserting and tightening the screw.
Bibliography
- Kaiser MG, Haid RW Jr, Subach BR, et al (2002) Anterior cervical plating enhances arthrodesis after discectomy and fusion with cortical allograft. Neurosurgery; 50(2):229236.
- Fraser JF, Hrtl R (2007) Anterior approaches to fusion of the cervical spine: a metaanalysis of fusion rates. J Neurosurg Spine; 6(4):298303.
- Scholz M, Reyes PM, Schleier P, et al (2009) A new stand-alone cervical anterior interbody fusion device: biomechanical comparison with established anterior cervical fixation devices. Spine; 34(2):156160.
Case provided by Frank Kandziora, Frankfurt, Germany
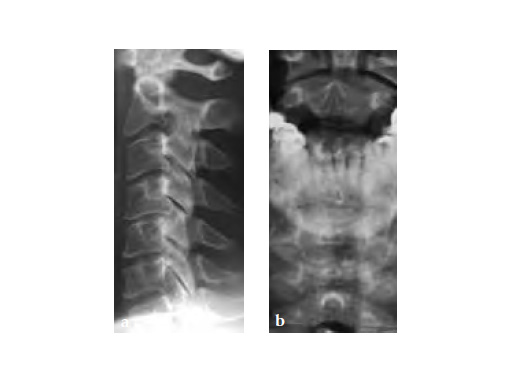
59-year-old female with neck pain and right radicular arm pain C5 and C6 and weakness during walking. An MRI was performed and a severe DDD with soft spinal stenosis C3C6 was diagnosed. Neurophysiology revealed myelopathic spinal cord changes. Decompression and stabilization C3/4, C4/5, and C5/6 using Zero-P was performed. After surgery the patient was nearly free of pain, had no complains regarding dysphagia, and was neurologically improved.
Hazards and labeling
Due to varying countries’ legal and regulatory approval requirements, consult the appropriate local product labeling for approved intended use of the products described on this website. All devices on this website are approved by the AO Technical Commission. For logistical reasons, these devices may not be available in all countries worldwide at the date of publication.
Legal restrictions
This work was produced by AO Foundation, Switzerland. All rights reserved by AO Foundation. This publication, including all parts thereof, is legally protected by copyright.
Any use, exploitation or commercialization outside the narrow limits set forth by copyright legislation and the restrictions on use laid out below, without the publisher‘s consent, is illegal and liable to prosecution. This applies in particular to photostat reproduction, copying, scanning or duplication of any kind, translation, preparation of microfilms, electronic data processing, and storage such as making this publication available on Intranet or Internet.
Some of the products, names, instruments, treatments, logos, designs, etc referred to in this publication are also protected by patents, trademarks or by other intellectual property protection laws (eg, “AO” and the AO logo are subject to trademark applications/registrations) even though specific reference to this fact is not always made in the text. Therefore, the appearance of a name, instrument, etc without designation as proprietary is not to be construed as a representation by the publisher that it is in the public domain.
Restrictions on use: The rightful owner of an authorized copy of this work may use it for educational and research purposes only. Single images or illustrations may be copied for research or educational purposes only. The images or illustrations may not be altered in any way and need to carry the following statement of origin “Copyright by AO Foundation, Switzerland”.
Check www.aofoundation.org/disclaimer for more information.
If you have any comments or questions on the articles or the new devices, please do not hesitate to contact us.
“approved by AO Technical Commission” and “approved by AO”
The brands and labels “approved by AO Technical Commission” and “approved by AO”, particularly "AO" and the AO logo, are AO Foundation's intellectual property and subject to trademark applications and registrations, respectively. The use of these brands and labels is regulated by licensing agreements between AO Foundation and the producers of innovation products obliged to use such labels to declare the products as AO Technical Commission or AO Foundation approved solutions. Any unauthorized or inadequate use of these trademarks may be subject to legal action.
AO ITC Innovations Magazine
Find all issues of the AO ITC Innovations Magazine for download here.
Innovation Awards
Recognizing outstanding achievements in development and fostering excellence in surgical innovation.