Vertebral Body Stent (VBS)
Paul F Heini, Cornelius Wimmer, Thomas Pfandlsteiner, Rick Bransford
The vertebral body stent (VBS) system is an efficient method to treat painful, traumatic, and osteoporotic compression fractures, as well as osteolytic lesions. It addresses the procedural drawbacks of common and established treatment options like vertebro- and kyphoplasty which can result in either incomplete fracture reposition or in intraoperative loss of the restored vertebral body height [1].
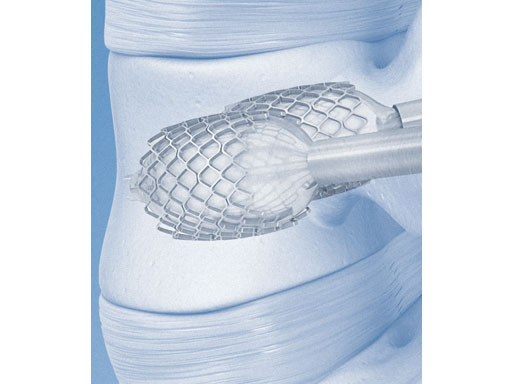
The VBS is an expandable metal scaffolding (ie, a stent) which can be inflated from inside a vertebral body. Stent technology has been known for two decades to treat vascular diseases [2]. In 2002 Sebastian Fürderer first published the idea of implanting two balloon-expandable stents into fractured vertebral bodies [3], which has also been biomechanically assessed in comparison to simple balloon kyphoplasty [4]. The technology has been available since 2008 and has been applied in more than 4,000 levels.
VBS advantages
The surgical technique of VBS involves a unique percutaneous minimally invasive procedure where the vertebral body can be accessed posteriorly. The stents are implanted laterally on both sides of the spine. After positioning, the stents can be balloon-expanded inside the collapsed vertebral body restoring its original height and the natural curvature of the spine. The stents will remain in the created cavity and prevent the recollapse of the fractured vertebra after the balloons are deflated and removed. The remaining cavities can then be safely filled using a PMMA-based bone cement, resulting in pain relief as well as quick and easy patient mobilization [5, 6].
References
- Feltes C, Fountas KN, Machinis T, et al (2005) Immediate and early postoperative pain relief after kyphoplasty without significant restoration of vertebral body height in acute osteoporotic vertebral fractures. Neurosurg Focus; 18(3):e5.
- [No authors listed] (2002) A Survey of Stent Designs. Minim Invas Ther Allied Technol; 11(4):137147.
- Fürderer S, Anders M, Schwindling B, et al (2002) [Vertebral body stenting. A method for repositioning and augmenting vertebral body compression fractures.] Orthopde; 31(4):356361. German.
- Rotter R, Martin H, Fuerderer S, et al (2010) Vertebral body stenting: a new method for vertebral augmentation versus kyphoplasty. Eur Spine J; 19 (6):916923.
- Alvarez L, Alkcaraz M, Prez-Higueras A, et al (2006) Percutaneous vertebroplasty: functional improvement in patients with osteoporotic compression fractures. Spine; 31(10):11131118.
- Ledlie JT, Renfro MB (2006) Kyphoplasty treatment of vertebral fractures: 2-year outcomes show sustained benefits. Spine; 31(1):5764.
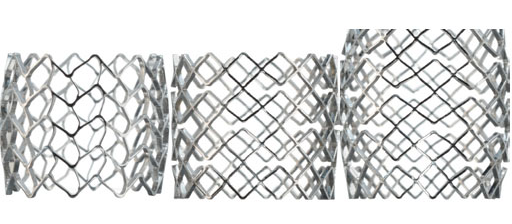
Instruments and stents
The new VBS small/medium/large (S/M/L) features a complete set of different stent sizes. With the available stent lengths of 13, 15, and 20 mm, it is possible to treat levels from T5 to L5 in a comprehensive range of patients anatomies.
All instruments are sterile packed, single use, and allow para- and transpedicular access either using a guide (K-) wire or a trocar. The setincludes a drill and plunger for clear intraoperative determination ofthe stent size. The cement injection cannula is designed with a special side opening for safe direction of the cement within the cavity.
One solution
VBS S/M/L can be used with vertecem V+ which is a PMMA-based bone cement ready to use right after mixing for up to 27 minutes. This allows maximum flexibility during surgery and an optimum use of OR time.
Case provided by Paul Heini, Bern, Switzerland
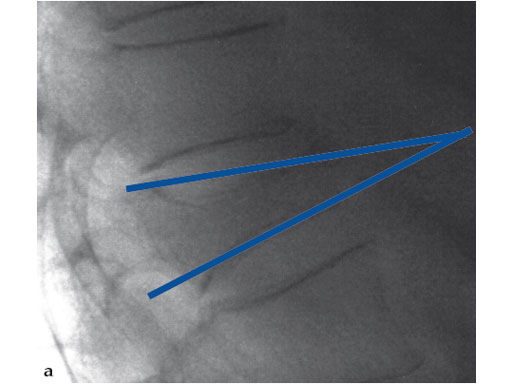
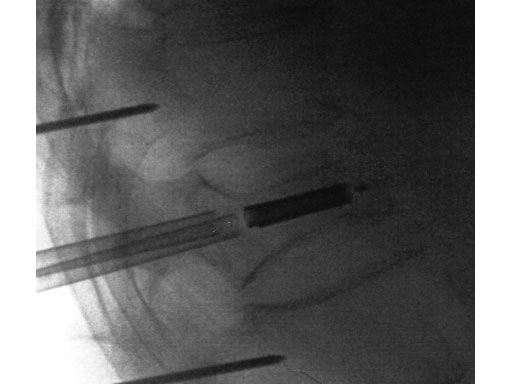
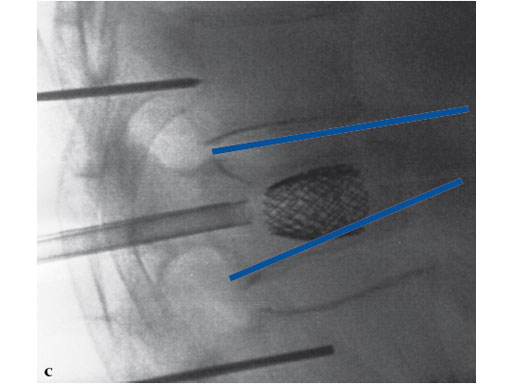
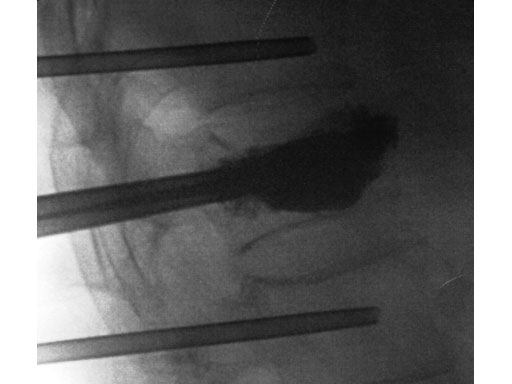
A 78-year-old man with back pain after a simple fall. An MRI showed a subacute fracture with a collapsed vertebral body of L1. A VB S was used for height restoration. The patient was pain-free immediately after the intervention.
Fig 1-4 Intraoperative images.
Hazards and labeling
Due to varying countries’ legal and regulatory approval requirements, consult the appropriate local product labeling for approved intended use of the products described on this website. All devices on this website are approved by the AO Technical Commission. For logistical reasons, these devices may not be available in all countries worldwide at the date of publication.
Legal restrictions
This work was produced by AO Foundation, Switzerland. All rights reserved by AO Foundation. This publication, including all parts thereof, is legally protected by copyright.
Any use, exploitation or commercialization outside the narrow limits set forth by copyright legislation and the restrictions on use laid out below, without the publisher‘s consent, is illegal and liable to prosecution. This applies in particular to photostat reproduction, copying, scanning or duplication of any kind, translation, preparation of microfilms, electronic data processing, and storage such as making this publication available on Intranet or Internet.
Some of the products, names, instruments, treatments, logos, designs, etc referred to in this publication are also protected by patents, trademarks or by other intellectual property protection laws (eg, “AO” and the AO logo are subject to trademark applications/registrations) even though specific reference to this fact is not always made in the text. Therefore, the appearance of a name, instrument, etc without designation as proprietary is not to be construed as a representation by the publisher that it is in the public domain.
Restrictions on use: The rightful owner of an authorized copy of this work may use it for educational and research purposes only. Single images or illustrations may be copied for research or educational purposes only. The images or illustrations may not be altered in any way and need to carry the following statement of origin “Copyright by AO Foundation, Switzerland”.
Check www.aofoundation.org/disclaimer for more information.
If you have any comments or questions on the articles or the new devices, please do not hesitate to contact us.
“approved by AO Technical Commission” and “approved by AO”
The brands and labels “approved by AO Technical Commission” and “approved by AO”, particularly "AO" and the AO logo, are AO Foundation's intellectual property and subject to trademark applications and registrations, respectively. The use of these brands and labels is regulated by licensing agreements between AO Foundation and the producers of innovation products obliged to use such labels to declare the products as AO Technical Commission or AO Foundation approved solutions. Any unauthorized or inadequate use of these trademarks may be subject to legal action.
AO ITC Innovations Magazine
Find all issues of the AO ITC Innovations Magazine for download here.
Innovation Awards
Recognizing outstanding achievements in development and fostering excellence in surgical innovation.