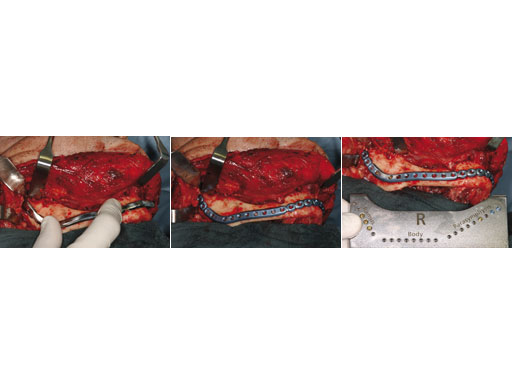
Matrix Mandible SystemPreformed Reconstruction Plate
One of the biggest challenges in mandibular reconstructive procedures is the bending of the large (or heavy) reconstruction plates. Not only can this become a time-consuming process, the bending tools can also introduce stress into the plate and even leave marks. Oftentimes this can lead to reduced fatigue life of the implant. The new preformed reconstruction plate was designed based on the matrix mandible systems 2.5 mm (light blue) plates to overcome the effects of plate fatigue due to overinstrumentation.
The preformed plates provide a 3-D shape which is based on the statistical analysis of mandible models obtained from over 2,000 CT scans, originating from various adult populations in collaboration with Marc C Metzger (Freiburg, Germany) who had already played a crucial part in developing the preformed orbital plate from the matrix orbit system.
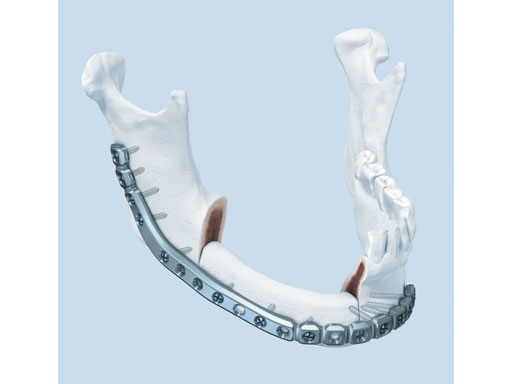
Fig 1 Preformed reconstruction plate.
The plates are available in three sizes: small, medium, and large with each plate offering a section of increased strength in the body and angle regions. The anatomical shape of the preformed reconstruction plates also allows for transoral application, ie, in combination with transbuccal instrumentation and/or the 90 screwdriver. The minimal intraoperative bending that is required preserves the optimal threaded-hole shape, especially in the preformed sections. These features result in a plate with increased fatigue life compared to standard reconstruction plates, thus reducing the risk of plate failure.
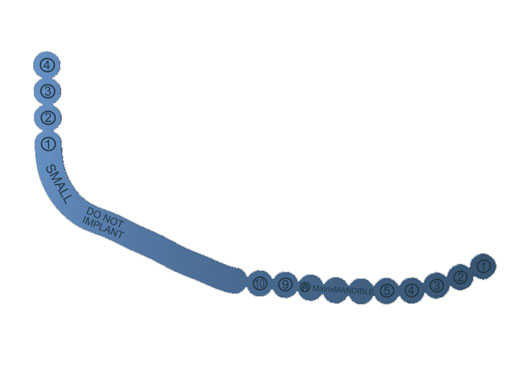
Fig 2 Set module.
The preformed mandible reconstruction plates are intended for use in oral and maxillofacial surgery, trauma, and reconstructive surgery. This includes primary mandibular reconstruction, comminuted fractures, and temporary bridging until delayed secondary reconstruction, including fractures in edentulous and atrophic mandibles, and unstable fractures. They are 2.5 mm thick and are made from pure titanium. They can be used with the light blue locking screws (2.4 mm) from the matrix mandible system. Anatomically preformed sizers facilitate the correct plate size selection in the OR.
Fig 3 Plate with screw module indicating the right screws for ramus, body, and symphysis.Fig 4 Sizer.
Fig 5 Bending template.
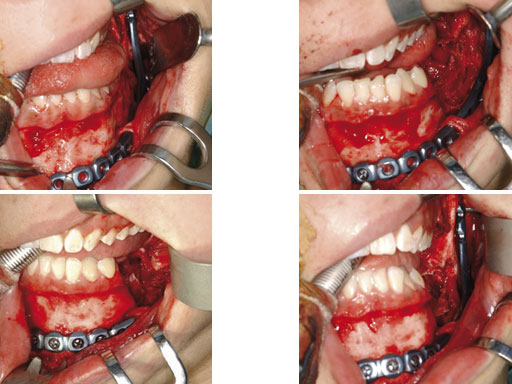
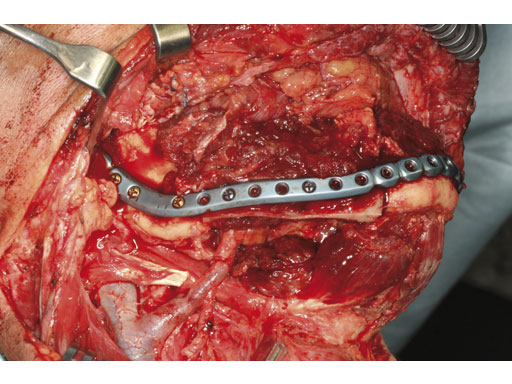
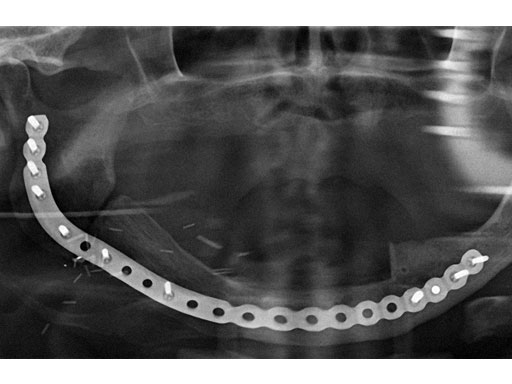
Case 1: A 27-year-old woman with an odontogenic myxoma in the left mandible. The preformed reconstruction plate was inserted prior to the tumor resection using a transoral approach with only a small additional transbuccal incision. To date the patient is free of symptoms with no signs of hypoaesthesia of the mandible and no signs of relapse. After another relapse-free period dental implants are planned for oral rehabilitation.
Fig 1 ad Transoral application of preformed reconstruction plate before tumor dissection.Fig 2
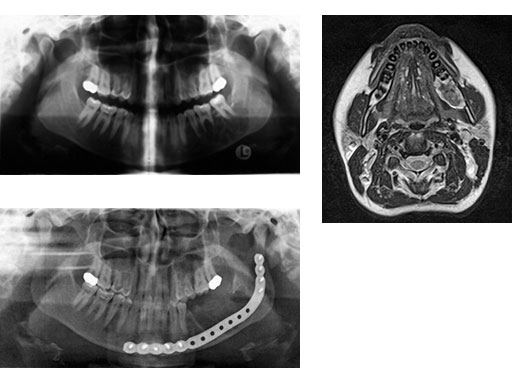
ab Preoperative radiograph and MRI.
c Postoperative radiograph.
Case provided by Christoph Pautke, Mnchen, Germany
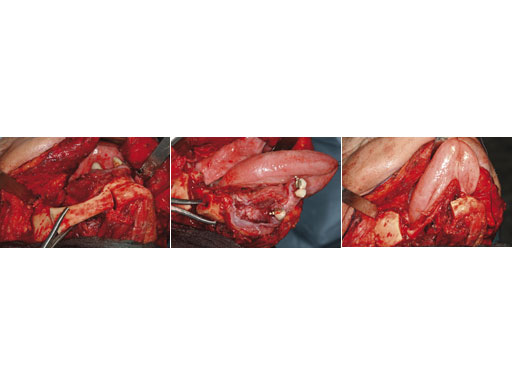
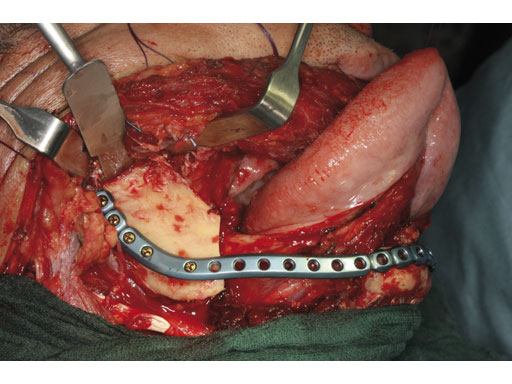
Case 2: A 65-year-old man suffering from an oral cancer in the anterolateral floor of the mouth with infiltration of the right mandible. The preformed reconstruction plate was applied to the lateral surface of the hemimandible prior to en bloc tumor resection, including a bone segment via extended submandibular access. The missing bone was replaced with a revascularized scapula border in combination with a soft-tissue parascapular flap. The patient has had no recurrence 1.5 years postoperatively.
Fig 1 Implant, sizer, and template on surgical field.
Fig 2ac Determining the correct implant size with the sizer and preliminary fixation.
Fig 3ac En bloc resection of the mandible body segment with the overlying soft tissues of the floor of the mouth.
Fig 4 Primary fixation of implant, serving as a load-bearing bridge over the bone gap.
Fig 5 Reconstruction with revascularized scapular border composite flap.
Case provided by Carl-Peter Cornelius, Mnchen, Germany
Hazards and labeling
Due to varying countries’ legal and regulatory approval requirements, consult the appropriate local product labeling for approved intended use of the products described on this website. All devices on this website are approved by the AO Technical Commission. For logistical reasons, these devices may not be available in all countries worldwide at the date of publication.
Legal restrictions
This work was produced by AO Foundation, Switzerland. All rights reserved by AO Foundation. This publication, including all parts thereof, is legally protected by copyright.
Any use, exploitation or commercialization outside the narrow limits set forth by copyright legislation and the restrictions on use laid out below, without the publisher‘s consent, is illegal and liable to prosecution. This applies in particular to photostat reproduction, copying, scanning or duplication of any kind, translation, preparation of microfilms, electronic data processing, and storage such as making this publication available on Intranet or Internet.
Some of the products, names, instruments, treatments, logos, designs, etc referred to in this publication are also protected by patents, trademarks or by other intellectual property protection laws (eg, “AO” and the AO logo are subject to trademark applications/registrations) even though specific reference to this fact is not always made in the text. Therefore, the appearance of a name, instrument, etc without designation as proprietary is not to be construed as a representation by the publisher that it is in the public domain.
Restrictions on use: The rightful owner of an authorized copy of this work may use it for educational and research purposes only. Single images or illustrations may be copied for research or educational purposes only. The images or illustrations may not be altered in any way and need to carry the following statement of origin “Copyright by AO Foundation, Switzerland”.
Check www.aofoundation.org/disclaimer for more information.
If you have any comments or questions on the articles or the new devices, please do not hesitate to contact us.
“approved by AO Technical Commission” and “approved by AO”
The brands and labels “approved by AO Technical Commission” and “approved by AO”, particularly "AO" and the AO logo, are AO Foundation's intellectual property and subject to trademark applications and registrations, respectively. The use of these brands and labels is regulated by licensing agreements between AO Foundation and the producers of innovation products obliged to use such labels to declare the products as AO Technical Commission or AO Foundation approved solutions. Any unauthorized or inadequate use of these trademarks may be subject to legal action.
AO ITC Innovations Magazine
Find all issues of the AO ITC Innovations Magazine for download here.
Innovation Awards
Recognizing outstanding achievements in development and fostering excellence in surgical innovation.