In-Space Interspinous Distraction
With the growth of the aging population, the incidence of lumbar spinal stenosis continues to increase. Decompression of the affected spinal level may be performed to relieve pressure off the spinal cord or nerve roots.
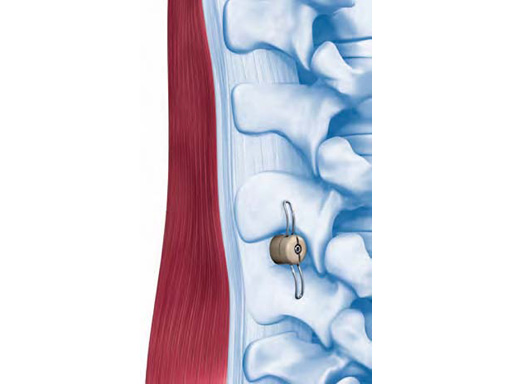
As a less invasive alternative for suitable patients in the form of interspinous devices are available to act as an extension stopper, since it is this motion that places additional pressure on the affected neural structures and causes pain. Patients with central, lateral and foraminal lumbar spinal stenosis with leg, buttock, or groin pain, which can be relieved during flexion, are good candidates for this procedure.
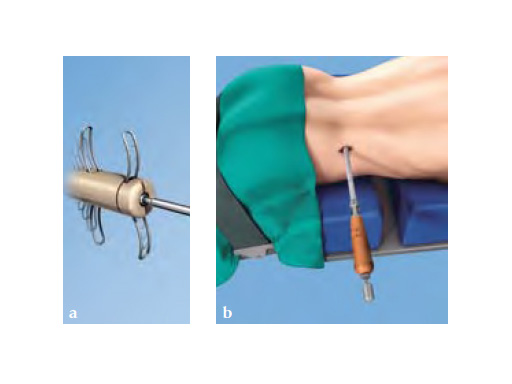
For the percutaneous approach, a guide wire is inserted to the spinal level that needs to be distracted. Next, distractors are placed sequentially one over the other until adequate distraction is reached. Finally, the implant is inserted and wings are deployed to help keep the implant in place.
With the posterior, mini-open approach, muscles only need to be stripped unilaterally, therefore it is less invasive than some competitors bilateral approach. Trial distractors are placed sequentially one after the other until adequate distraction is reached. The final step, as with the lateral approach, is to insert the implant and deploy the wings.
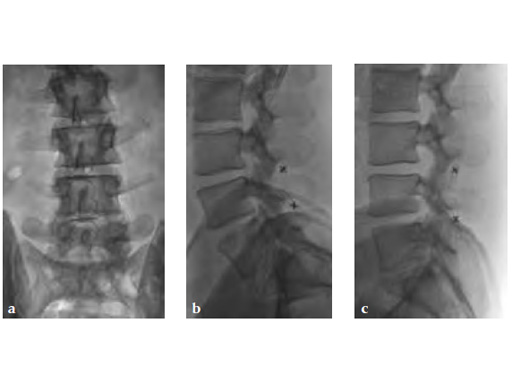
Both surgical approaches are aided by x-ray imaging, to better visualize proper placement of the in-space. Unlike standard decompression surgery, in-space does not require the removal of any bone and supporting ligaments remain uncompromised. Thus, spinal stability is not affected by the procedure.
A single level in-space surgery is typically performed in 15-35 minutes, significantly less compared to decompression surgery. Therefore, and due to the minimally invasive nature of the procedure, patients tend to experience less postoperative pain, minimal bleeding, and a shorter hospital stay, followed by a quicker return to daily activities.
It is important to note that interspinous technology is not a cure-all. It is only one step in the continuum of surgical treatment for degenerative spinal disorders.
Case provided by Paul W Pavlov, Nijmegen, The Netherlands
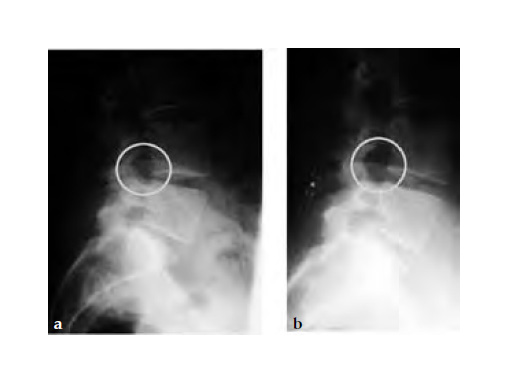
32-year-old male with neurogenic claudication due to spinal stenosis (degenerative bulging discs). Complete relief of symptoms.
Hazards and labeling
Due to varying countries’ legal and regulatory approval requirements, consult the appropriate local product labeling for approved intended use of the products described on this website. All devices on this website are approved by the AO Technical Commission. For logistical reasons, these devices may not be available in all countries worldwide at the date of publication.
Legal restrictions
This work was produced by AO Foundation, Switzerland. All rights reserved by AO Foundation. This publication, including all parts thereof, is legally protected by copyright.
Any use, exploitation or commercialization outside the narrow limits set forth by copyright legislation and the restrictions on use laid out below, without the publisher‘s consent, is illegal and liable to prosecution. This applies in particular to photostat reproduction, copying, scanning or duplication of any kind, translation, preparation of microfilms, electronic data processing, and storage such as making this publication available on Intranet or Internet.
Some of the products, names, instruments, treatments, logos, designs, etc referred to in this publication are also protected by patents, trademarks or by other intellectual property protection laws (eg, “AO” and the AO logo are subject to trademark applications/registrations) even though specific reference to this fact is not always made in the text. Therefore, the appearance of a name, instrument, etc without designation as proprietary is not to be construed as a representation by the publisher that it is in the public domain.
Restrictions on use: The rightful owner of an authorized copy of this work may use it for educational and research purposes only. Single images or illustrations may be copied for research or educational purposes only. The images or illustrations may not be altered in any way and need to carry the following statement of origin “Copyright by AO Foundation, Switzerland”.
Check www.aofoundation.org/disclaimer for more information.
If you have any comments or questions on the articles or the new devices, please do not hesitate to contact us.
“approved by AO Technical Commission” and “approved by AO”
The brands and labels “approved by AO Technical Commission” and “approved by AO”, particularly "AO" and the AO logo, are AO Foundation's intellectual property and subject to trademark applications and registrations, respectively. The use of these brands and labels is regulated by licensing agreements between AO Foundation and the producers of innovation products obliged to use such labels to declare the products as AO Technical Commission or AO Foundation approved solutions. Any unauthorized or inadequate use of these trademarks may be subject to legal action.
AO ITC Innovations Magazine
Find all issues of the AO ITC Innovations Magazine for download here.
Innovation Awards
Recognizing outstanding achievements in development and fostering excellence in surgical innovation.