3.5 mm LCP Low Bend Medial Distal Tibia
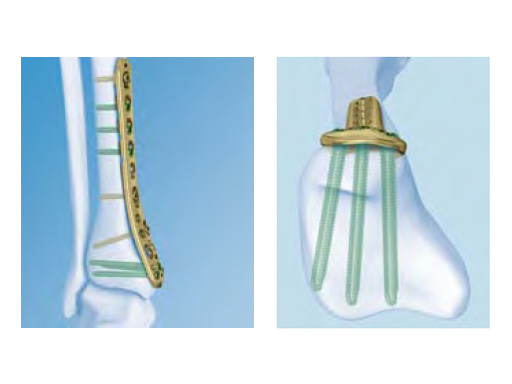
The design of the 3.5 mm LCP low bend medial distal tibia focuses on the relationship between the distal plate tip and the medial malleolus. A lower head height (minus 1.96 mm) compared to the current 3.5 LCP medial distal tibia increases contact with the crest of the medial malleolus. The shaft twist is more gradual and has a higher bend distance of 70 mm compared to 44 mm in the previous implant (measured from where the twist begins to the distal tip). The larger radius of curvature (R125 compared to R58) and the higher starting point leads to a smoother transition. It has the same screw hole configuration and pattern as the existing plate, except for the articulated tension device hole which was removed.
Cadaver tests showed that the low bend design, especially the metaphyseal bend, has a better fit on the average tibia. The plate has a left and right version and is available in stainless steel and titanium. It comes in lengths from 4 holes (109 mm) up to 14 holes (239 mm).
LCP Small Fragment Percutaneous Instrument Set
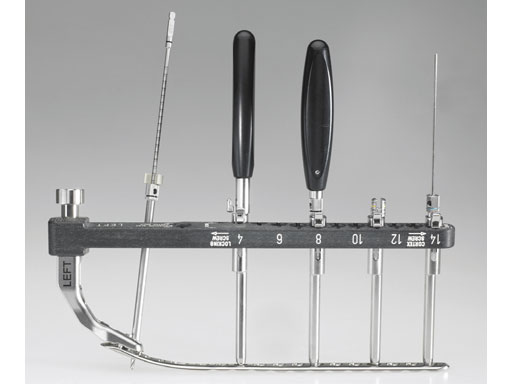
The LCP small fragment percutaneous instrument set consists of a comprehensive series of aiming arms, insertion handles, and instrumentation to facilitate percutaneous, subcutaneous/submuscular insertion of various plates to allow minimally invasive surgery for reduced soft-tissue damage. It may also reduce image intensifier exposure and time in the OR because the arm targets the holes and facilitates screw insertion.
The set enables aiming for all three positions of the combination hole (locking, neutral, and compression). The instruments snap into the aiming arms for quick assembly and removal. Color coding helps easy identification of compatible instruments. The aiming arm is made of carbon fiber for radiolucency. The insertion handle attachment point provides three points of fixation when connected to the plate.
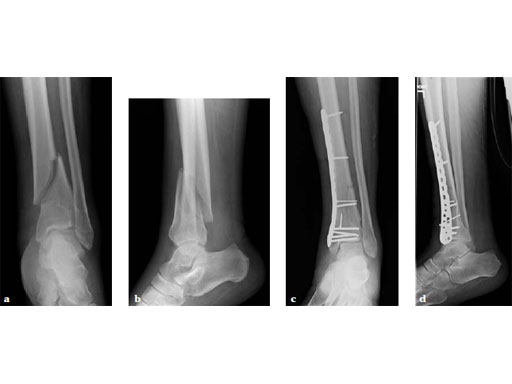
75-year-old male, post motor vehicle collision with ipsilateral split depression lateral tibial plateau fracture.
Case provided by Matthew Graves, Jackson, USA
Hazards and labeling
Due to varying countries’ legal and regulatory approval requirements, consult the appropriate local product labeling for approved intended use of the products described on this website. All devices on this website are approved by the AO Technical Commission. For logistical reasons, these devices may not be available in all countries worldwide at the date of publication.
Legal restrictions
This work was produced by AO Foundation, Switzerland. All rights reserved by AO Foundation. This publication, including all parts thereof, is legally protected by copyright.
Any use, exploitation or commercialization outside the narrow limits set forth by copyright legislation and the restrictions on use laid out below, without the publisher‘s consent, is illegal and liable to prosecution. This applies in particular to photostat reproduction, copying, scanning or duplication of any kind, translation, preparation of microfilms, electronic data processing, and storage such as making this publication available on Intranet or Internet.
Some of the products, names, instruments, treatments, logos, designs, etc referred to in this publication are also protected by patents, trademarks or by other intellectual property protection laws (eg, “AO” and the AO logo are subject to trademark applications/registrations) even though specific reference to this fact is not always made in the text. Therefore, the appearance of a name, instrument, etc without designation as proprietary is not to be construed as a representation by the publisher that it is in the public domain.
Restrictions on use: The rightful owner of an authorized copy of this work may use it for educational and research purposes only. Single images or illustrations may be copied for research or educational purposes only. The images or illustrations may not be altered in any way and need to carry the following statement of origin “Copyright by AO Foundation, Switzerland”.
Check www.aofoundation.org/disclaimer for more information.
If you have any comments or questions on the articles or the new devices, please do not hesitate to contact us.
“approved by AO Technical Commission” and “approved by AO”
The brands and labels “approved by AO Technical Commission” and “approved by AO”, particularly "AO" and the AO logo, are AO Foundation's intellectual property and subject to trademark applications and registrations, respectively. The use of these brands and labels is regulated by licensing agreements between AO Foundation and the producers of innovation products obliged to use such labels to declare the products as AO Technical Commission or AO Foundation approved solutions. Any unauthorized or inadequate use of these trademarks may be subject to legal action.
AO ITC Innovations Magazine
Find all issues of the AO ITC Innovations Magazine for download here.
Innovation Awards
Recognizing outstanding achievements in development and fostering excellence in surgical innovation.