2.7/3.5 mm LCP Posterolateral Distal Fibula
Treating unstable ankle fractures successfully requires anatomical reduction and stabilization of the distal fibula. This involves restoring accurate length, alignment, and rotation. Often this may be complicated by comminution, osteoporotic bone, and associated syndesmotic instability. Laterally placed fibula plates do not allow for buttressing of the common posterior or posterolateral fracture spike of the distal fragment, and positioning of the plate is directly subcutaneous to the incision often through thin and compromised skin. Using standard small fragment implants provides limited options for distal fixation.
The LCP posterolateral distal fibula plate offers six round locking holes and two coaxial holes distally which accept 2.4 and 2.7 mm locking and cortex screws to provide multiple screw options. The distal holes are slightly divergent to help prevent screw pullout. The coaxial hole accepts both locking and cortex screws and its recess for screw heads minimizes screw-head prominence by allowing the screws to sit more flush with the plate, creating a low-profile construct. Its posterolateral position allows it to be placed deep to the peroneal muscles, minimizing the risk of wound healing problems due to prominence. A 2.7 mm lag screw may be placed through the distal screw cluster, and a syndesmotic screw may also be placed through the plate.
The anatomically precontoured plate shaft is only 2.25 mm thick yet substantially stronger than the one-third tubular plate. The combination holes in the shaft accept 3.5 mm locking screws, 3.5 mm cortex screws, and 4.0 mm cancellous bone screws.
The plate comes in left and right versions and is available in lengths from 77233 mm (3, 4, 5, 6, 7, 9, 11, 13, and 15 holes). It is offered in stainless steel and titanium, sterile, and nonsterile.
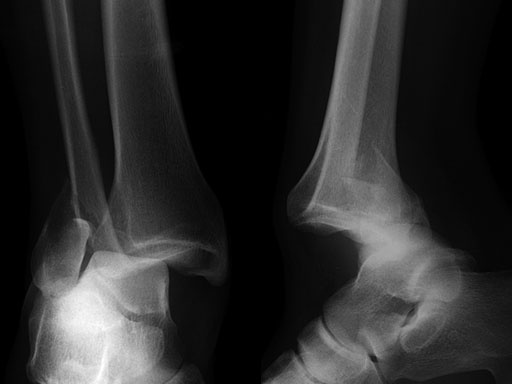
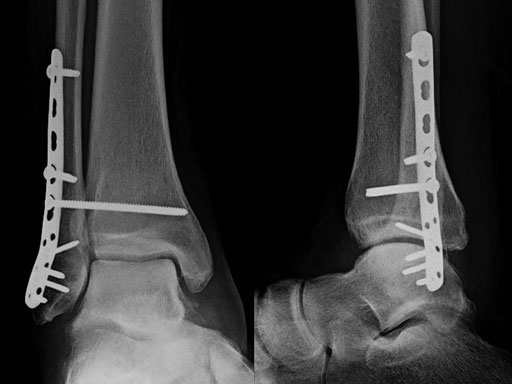
Case provided by Michael J Gardner, St Louis, USA.
A 56-year-old man slipped and fell, sustaining a fracture dislocation of the ankle. His fibula was stabilized with a posterolateral plate. The construct included several nonlocking screws in the diaphysis, and multiple 2.7 mm locking screws in the distal fibula. As with most Weber B fracture patterns, a lag screw was possible from posterior to anterior through the plate. Finally, intraoperatively the patient was found to have a syndesmotic injury, and after reduction, a syndesmotic screw was placed through the plate.
Hazards and labeling
Due to varying countries’ legal and regulatory approval requirements, consult the appropriate local product labeling for approved intended use of the products described on this website. All devices on this website are approved by the AO Technical Commission. For logistical reasons, these devices may not be available in all countries worldwide at the date of publication.
Legal restrictions
This work was produced by AO Foundation, Switzerland. All rights reserved by AO Foundation. This publication, including all parts thereof, is legally protected by copyright.
Any use, exploitation or commercialization outside the narrow limits set forth by copyright legislation and the restrictions on use laid out below, without the publisher‘s consent, is illegal and liable to prosecution. This applies in particular to photostat reproduction, copying, scanning or duplication of any kind, translation, preparation of microfilms, electronic data processing, and storage such as making this publication available on Intranet or Internet.
Some of the products, names, instruments, treatments, logos, designs, etc referred to in this publication are also protected by patents, trademarks or by other intellectual property protection laws (eg, “AO” and the AO logo are subject to trademark applications/registrations) even though specific reference to this fact is not always made in the text. Therefore, the appearance of a name, instrument, etc without designation as proprietary is not to be construed as a representation by the publisher that it is in the public domain.
Restrictions on use: The rightful owner of an authorized copy of this work may use it for educational and research purposes only. Single images or illustrations may be copied for research or educational purposes only. The images or illustrations may not be altered in any way and need to carry the following statement of origin “Copyright by AO Foundation, Switzerland”.
Check www.aofoundation.org/disclaimer for more information.
If you have any comments or questions on the articles or the new devices, please do not hesitate to contact us.
“approved by AO Technical Commission” and “approved by AO”
The brands and labels “approved by AO Technical Commission” and “approved by AO”, particularly "AO" and the AO logo, are AO Foundation's intellectual property and subject to trademark applications and registrations, respectively. The use of these brands and labels is regulated by licensing agreements between AO Foundation and the producers of innovation products obliged to use such labels to declare the products as AO Technical Commission or AO Foundation approved solutions. Any unauthorized or inadequate use of these trademarks may be subject to legal action.
AO ITC Innovations Magazine
Find all issues of the AO ITC Innovations Magazine for download here.
Innovation Awards
Recognizing outstanding achievements in development and fostering excellence in surgical innovation.