2.7 mm LCP Pediatric Hip Plate
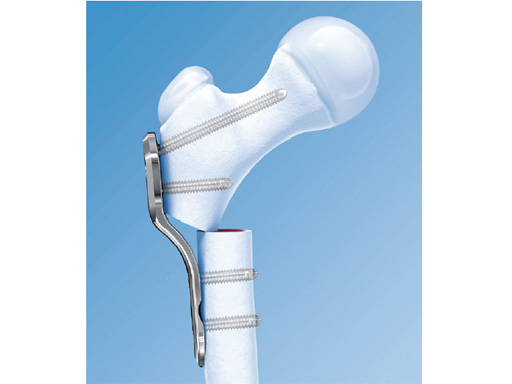
The 2.7 mm LCP pediatric hip plate is intended for use in infants up to 3 years, depending on weight, size, and bone quality. It is part of the LCP pediatric hip plate system for stable fixation of varus, valgus, and derotation osteotomies and fractures in pediatric orthopaedics. It incorporates the technique of the locking compression plate into a system dedicated to pediatrics.
This plate addresses the clinical problem of hip displacement in young patients as a result of late presentation or failed conservative management of developmental dysplasia of the hip (DDH).
Prior to the development of this plate the only solution for proximal femoral osteotomy in this group of patients was the infant angled blade plate based on a one-third tubular plate. The issues with this were weakness, poor rotational control, and the lack of guided insertion. The 3.5 mm LCP pediatric hip plates are too bulky for infants at the age required, which is generally around 18 months.
The 2.7 mm LCP pediatric hip plate was developed to close the gap for treatments of infants up to 3 years, depending on weight, size, and bone quality. The specific indications include:
- Neglected dislocation of the hip in combination with open reduction
- Idiopathic coxa valga
- Severe hip dysplasia
The implant completes the LCP pediatric plate portfolio with a full range of plates with a choice of angles. The locking compression functionality reduces the risk of a primary and secondary loss of correction. The plate can be used in locked internal fixator mode or as a compression plate as required. Although in the 3.5 and 5.0 mm LCP hip plates stability negates the need for external support, with the 2.7 mm plate a hip spica is recommended in all cases; the cast is frequently required for other elements of the surgery, such as open reduction of the hip.
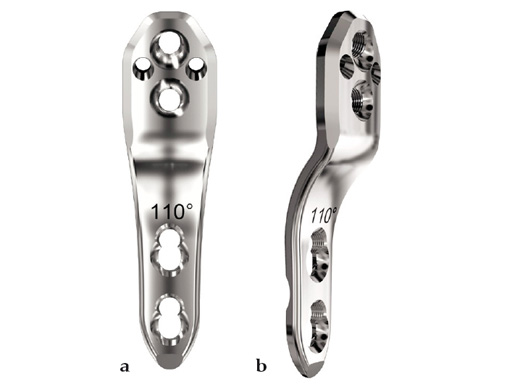
The 2.7 mm LCP pediatric hip plate is available with neck shaft angles of 100, 110 for varus osteotomies, and 130 for pure derotation osteotomies.
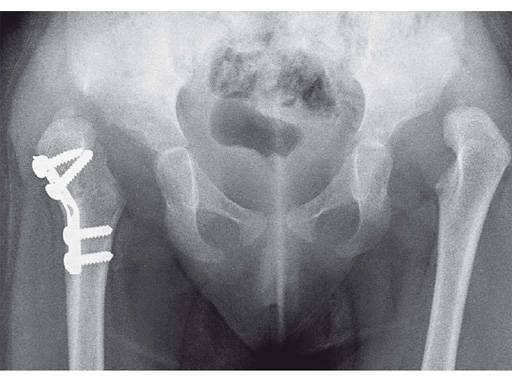
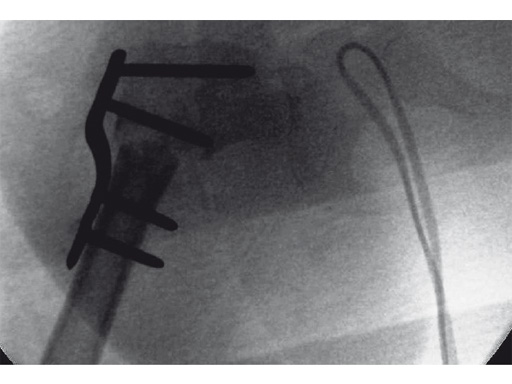
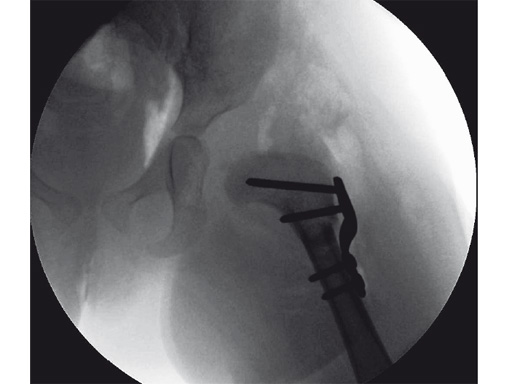
An 18-month old girl presented with developmental dysplasia of the hip. She had failed conservative management with a Pavlik harness, and redislocated following open reduction and femoral osteotomy of the right hip. She underwent revision open reduction and femoral osteotomy of the right hip, followed by the same procedure on the left hip 6 weeks later.
Case provided by James B Hunter, Nottingham, UK
Hazards and labeling
Due to varying countries’ legal and regulatory approval requirements, consult the appropriate local product labeling for approved intended use of the products described on this website. All devices on this website are approved by the AO Technical Commission. For logistical reasons, these devices may not be available in all countries worldwide at the date of publication.
Legal restrictions
This work was produced by AO Foundation, Switzerland. All rights reserved by AO Foundation. This publication, including all parts thereof, is legally protected by copyright.
Any use, exploitation or commercialization outside the narrow limits set forth by copyright legislation and the restrictions on use laid out below, without the publisher‘s consent, is illegal and liable to prosecution. This applies in particular to photostat reproduction, copying, scanning or duplication of any kind, translation, preparation of microfilms, electronic data processing, and storage such as making this publication available on Intranet or Internet.
Some of the products, names, instruments, treatments, logos, designs, etc referred to in this publication are also protected by patents, trademarks or by other intellectual property protection laws (eg, “AO” and the AO logo are subject to trademark applications/registrations) even though specific reference to this fact is not always made in the text. Therefore, the appearance of a name, instrument, etc without designation as proprietary is not to be construed as a representation by the publisher that it is in the public domain.
Restrictions on use: The rightful owner of an authorized copy of this work may use it for educational and research purposes only. Single images or illustrations may be copied for research or educational purposes only. The images or illustrations may not be altered in any way and need to carry the following statement of origin “Copyright by AO Foundation, Switzerland”.
Check www.aofoundation.org/disclaimer for more information.
If you have any comments or questions on the articles or the new devices, please do not hesitate to contact us.
“approved by AO Technical Commission” and “approved by AO”
The brands and labels “approved by AO Technical Commission” and “approved by AO”, particularly "AO" and the AO logo, are AO Foundation's intellectual property and subject to trademark applications and registrations, respectively. The use of these brands and labels is regulated by licensing agreements between AO Foundation and the producers of innovation products obliged to use such labels to declare the products as AO Technical Commission or AO Foundation approved solutions. Any unauthorized or inadequate use of these trademarks may be subject to legal action.
AO ITC Innovations Magazine
Find all issues of the AO ITC Innovations Magazine for download here.
Innovation Awards
Recognizing outstanding achievements in development and fostering excellence in surgical innovation.