1.3mm Pediatric Curvilinear Distractor
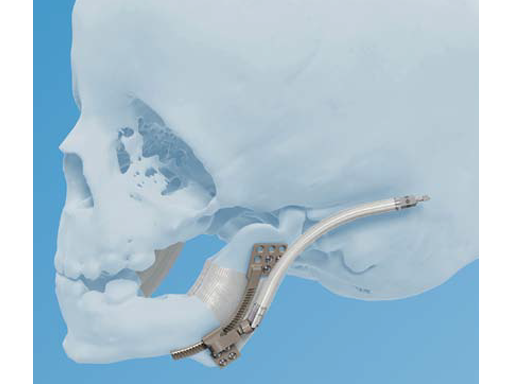
The 1.3 mm Pediatric Curvilinear Distractor (Fig 1) was developed to address an unmet clinical need for distraction devices in pediatric patients less than 1 year old. The 2.0mm system is indicated only for adults or pediatric patients over one year of age.
The new 1.3 mm Pediatric Curvilinear Distractor system (Fig 2) is an internal distraction osteogenesis device that gradually advances the mandible along a curved trajectory of distraction. As with the previously developed 2.0 mm Curvilinear Distractor, the 1.3 mm is indicated for correction of congenital deficiencies or posttraumatic defects on the mandibular body and ramus, where gradual bone distraction is required. This 1.3 mm pediatric version is intended for patients four years of age and younger.
The aim of curvilinear distraction is twofold: to lengthen the mandible in both vertical and horizontal planes, and to close, or avoid creating, an open bite.
In order to adapt the existing distractor's design to the smaller anatomy of pediatric patients, it was necessary to reduce the overall size of the device both in terms of profile (from 7.5 mm to 5.5 mm) and track width (from 4.25 mm to 3 mm). The device was named 2.0mm Curvilinear Distractor (Fig 3). Other modifications include the footplate design, which changed to a mesh pattern to allow for the insertion of more screws in a smaller area of bone, and the distractor acceptance of 1.3 mm diameter bone screws.
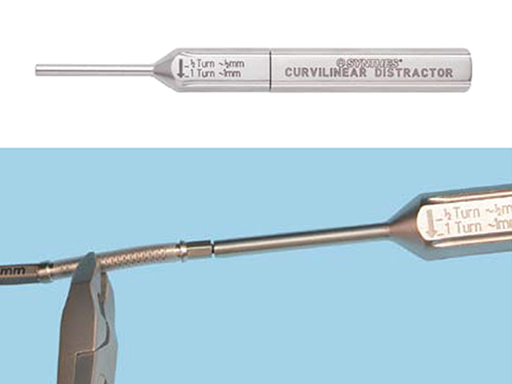
The device allows advancement of up to 35 mm, leaving the surgeon the option of cutting and crimping the track if less advancement is required. Existing tools, such as cutters and crimpers, were slightly modified to be able to work with both sized distractors: 2.0 mm and 1.3 mm (Fig 4).
A functional stop is created by a track crimp to avoid undesired device disassembling (Fig 5). The distractor is made of titanium molybdenum, is for single use only, and has left and right assemblies available in different radii of curvature (30 mm, 40 mm, 50 mm, 70 mm, and 100 mm), as well as a straight version.
All distractors accept removable extension arms (Fig 6), which move the point of activation to a location that is easily accessible with the activation instrument.
One full rotation of the activation instrument (Fig 7) equals 1.0 mm of distraction per day (one half turn twice daily), and is recommended to prevent premature consolidation. In young patients a distraction rate of 1.5 to 2.0 mm per day could be considered.
Hazards and labeling
Due to varying countries’ legal and regulatory approval requirements, consult the appropriate local product labeling for approved intended use of the products described on this website. All devices on this website are approved by the AO Technical Commission. For logistical reasons, these devices may not be available in all countries worldwide at the date of publication.
Legal restrictions
This work was produced by AO Foundation, Switzerland. All rights reserved by AO Foundation. This publication, including all parts thereof, is legally protected by copyright.
Any use, exploitation or commercialization outside the narrow limits set forth by copyright legislation and the restrictions on use laid out below, without the publisher‘s consent, is illegal and liable to prosecution. This applies in particular to photostat reproduction, copying, scanning or duplication of any kind, translation, preparation of microfilms, electronic data processing, and storage such as making this publication available on Intranet or Internet.
Some of the products, names, instruments, treatments, logos, designs, etc referred to in this publication are also protected by patents, trademarks or by other intellectual property protection laws (eg, “AO” and the AO logo are subject to trademark applications/registrations) even though specific reference to this fact is not always made in the text. Therefore, the appearance of a name, instrument, etc without designation as proprietary is not to be construed as a representation by the publisher that it is in the public domain.
Restrictions on use: The rightful owner of an authorized copy of this work may use it for educational and research purposes only. Single images or illustrations may be copied for research or educational purposes only. The images or illustrations may not be altered in any way and need to carry the following statement of origin “Copyright by AO Foundation, Switzerland”.
Check www.aofoundation.org/disclaimer for more information.
If you have any comments or questions on the articles or the new devices, please do not hesitate to contact us.
“approved by AO Technical Commission” and “approved by AO”
The brands and labels “approved by AO Technical Commission” and “approved by AO”, particularly "AO" and the AO logo, are AO Foundation's intellectual property and subject to trademark applications and registrations, respectively. The use of these brands and labels is regulated by licensing agreements between AO Foundation and the producers of innovation products obliged to use such labels to declare the products as AO Technical Commission or AO Foundation approved solutions. Any unauthorized or inadequate use of these trademarks may be subject to legal action.
AO ITC Innovations Magazine
Find all issues of the AO ITC Innovations Magazine for download here.
Innovation Awards
Recognizing outstanding achievements in development and fostering excellence in surgical innovation.