UTN PROtect (UTN, Gentamicin-Coated)
Despite improvements in the prophylactic and the therapeutic measures, soft-tissue damage and consecutive infection remain the major dilemma in the healing process of long bone fractures. Osteomyelitis is a consequence of local contamination by germs combined with a local and/or systemic immunodeficiency. Additionally, blood supply in the traumatized bone is disturbed which means systemic applied antibiotics do not reach the fractured region. In consequence, the further course may lead to severe disability including soft-tissue and/or segmental bone defects, impairment of joint motion, or even amputation. Once osteomyelitis has occurred, removal of the implant and aggressive debridement of the infected bone as well as soft tissue, combined with heavy local and systemic antibiotic therapy often represent the only option of treatment.
Bacteria introduced at the time of surgical implantation from the lower layers of the patients own skin which are not reached by skin disinfection, or at the time of injury in open fractures, compete with the patients immune system. This is often described as a race for the surface. Certain bacteria can colonize the surface of an implant and form a protective biofilm composed of proteins and polysaccharides, protecting the bacteria from the patients immune system as well as from the action of systemically applied antibiotics.
An antibiotic coating on an implant may prevent the bacteria from colonizing on the implants surface. In addition a coating with high local antimicrobial concentrations could protect the site of injury and avoid side effects resulting from long term high dose systemic antibiotic therapy.
The UTN PROtect combines the established UTN with a fully resorbable coating consisting of a fully amorphous polylactide (PDLLA) carrier containing gentamicin sulphate. Gentamicin was chosen due to its aminoglycoside, broad antibacterial spectrum, the bactericidal effect (non-proliferating bacteria), its synergistic effect in combination with cephalosporins, and because it is well established for local application in orthopedics (bone cement, PMMA-beads, collagen sponges). The total amount of antibiotic contained on one implant ranges from around 2040 mg, depending on the size of the implant. The gentamicin is released from the coating immediately after implantation with an initial burst that achieves a high peak concentration in the first hours and fades out over a period of 6 weeks. After coating the implant is packaged and sterilized by gamma irradiation and delivered sterile to the clinics.
The UTN PROtect will be the basis for all future generations of coated expert nails. A coated expert lateral femoral nail has already been used successfully as custom-made device.
Mechanical properties of the nail are not affected by the coating. Tests on cadavers and plastic bones proved the coating to be resistant to the abrasive forces present during insertion into a narrow and moist bone canal. The surgical technique does not differ from the regular standard of care in UTN implantation.
A clinical study run by AO Clinical Investigation and Documentation (AOCID) is ongoing. Preliminary data of the present results seem very encouraging, showing a superior outcome in comparison with published data. No adverse side effects due to the coated implant were detected. However, the study needs to complete before final results can be assessed.
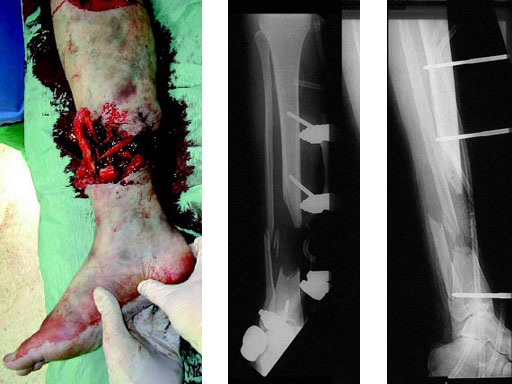
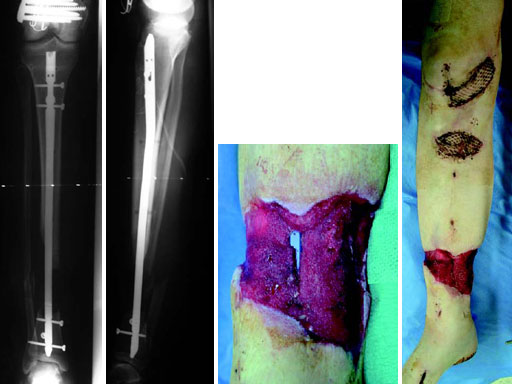
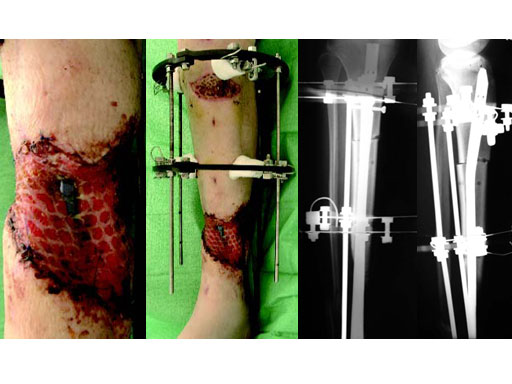
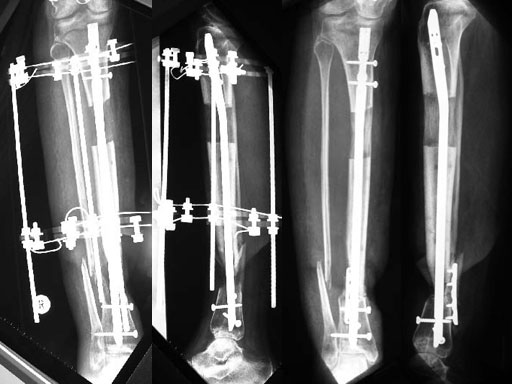
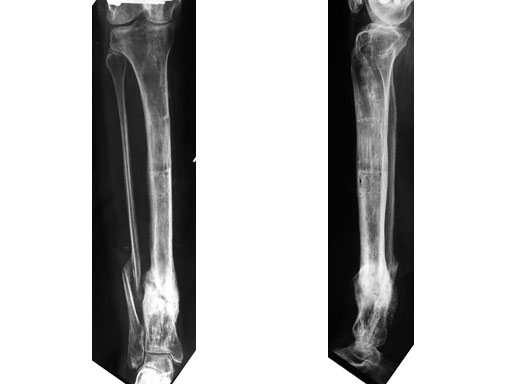
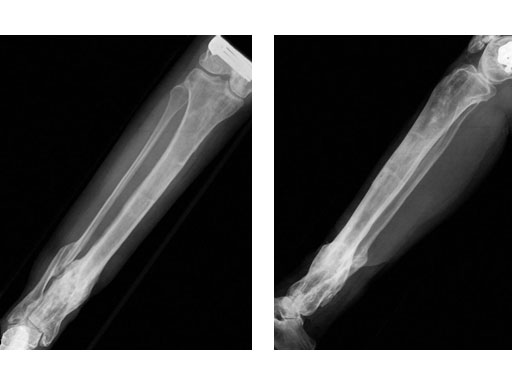
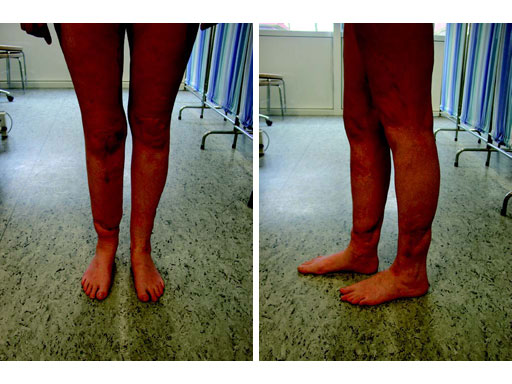
A 33-year-old female sustained a grade III open fracture of the right lower leg.
Case provided by Michael Raschke, Mnster, DE
Biomaterials in Infected Non-Unions
Antibiotic-coated nails in Trauma Surgery
Hazards and labeling
Due to varying countries’ legal and regulatory approval requirements, consult the appropriate local product labeling for approved intended use of the products described on this website. All devices on this website are approved by the AO Technical Commission. For logistical reasons, these devices may not be available in all countries worldwide at the date of publication.
Legal restrictions
This work was produced by AO Foundation, Switzerland. All rights reserved by AO Foundation. This publication, including all parts thereof, is legally protected by copyright.
Any use, exploitation or commercialization outside the narrow limits set forth by copyright legislation and the restrictions on use laid out below, without the publisher‘s consent, is illegal and liable to prosecution. This applies in particular to photostat reproduction, copying, scanning or duplication of any kind, translation, preparation of microfilms, electronic data processing, and storage such as making this publication available on Intranet or Internet.
Some of the products, names, instruments, treatments, logos, designs, etc referred to in this publication are also protected by patents, trademarks or by other intellectual property protection laws (eg, “AO” and the AO logo are subject to trademark applications/registrations) even though specific reference to this fact is not always made in the text. Therefore, the appearance of a name, instrument, etc without designation as proprietary is not to be construed as a representation by the publisher that it is in the public domain.
Restrictions on use: The rightful owner of an authorized copy of this work may use it for educational and research purposes only. Single images or illustrations may be copied for research or educational purposes only. The images or illustrations may not be altered in any way and need to carry the following statement of origin “Copyright by AO Foundation, Switzerland”.
Check www.aofoundation.org/disclaimer for more information.
If you have any comments or questions on the articles or the new devices, please do not hesitate to contact us.
“approved by AO Technical Commission” and “approved by AO”
The brands and labels “approved by AO Technical Commission” and “approved by AO”, particularly "AO" and the AO logo, are AO Foundation's intellectual property and subject to trademark applications and registrations, respectively. The use of these brands and labels is regulated by licensing agreements between AO Foundation and the producers of innovation products obliged to use such labels to declare the products as AO Technical Commission or AO Foundation approved solutions. Any unauthorized or inadequate use of these trademarks may be subject to legal action.
AO ITC Innovations Magazine
Find all issues of the AO ITC Innovations Magazine for download here.
Innovation Awards
Recognizing outstanding achievements in development and fostering excellence in surgical innovation.