MatrixRIB Fixation System
William Long, Mario Gasparri, Stephan Schulz-Drost, Arthur Martella, Edward Black
MatrixRIB - A comprehensive system
The new MatrixRIB Fixation System is indicated for the fixation and stabilization of rib fractures, fusions and osteotomies of normal and osteoporotic bone. It was first developed under the guidance of the Sternal Surgery Working Group, an international group of cardiothoracic and plastic surgeons within the CMF branch of the AOTK System.
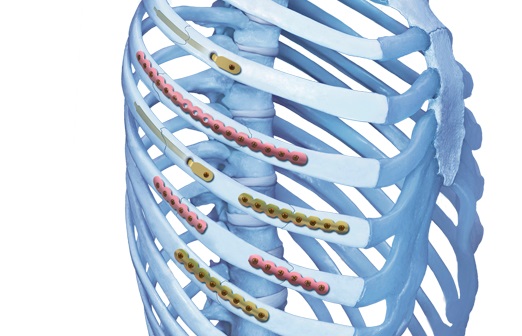
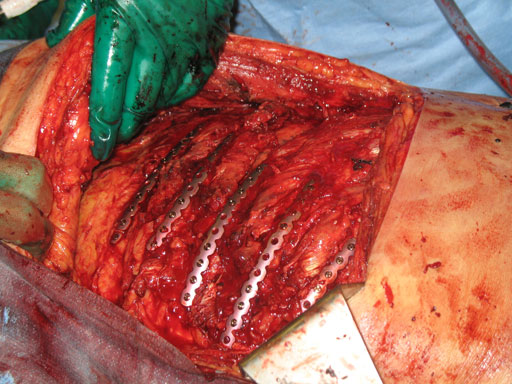
This new system is based on the Matrix platform of plates and screws that have been developed for all areas in CMF surgery. It consists of precontoured locking plates, locking screws, and intramedullary splints for the fixation and stabilization of ribs (Fig 1) and is indicated for the fixation and stabilization of rib fractures, fusions and osteotomies of normal and osteoporotic bone.
Reconstruction of the chest wall using metallic rib systems is gaining popularity. The AOTK Approved MatrixRIB system involves the use of plates and screws to bridge any defect and provide support for the chest wall following resection. It is a unique system offering stable fixation of normal and osteoporotic ribs combined with a minimally invasive technique. Although many rib fractures are treated conservatively, some patients benefit from surgical stabilization. The potential benefits of surgical intervention include reduced duration of mechanical ventilation support, shortened ICU stays and hospitalization, better secretion management through efficient cough and minimized chest wall deformities resulting from trauma.
The need to improve rib fracture care has been recognized for many years. To this end a number of surgeons have been using operative approaches including plates, intramedullary devices, vertical bridging, wire, sutures, and struts to repair the chest wall. Next to the attempt to achieve improvements in pain control, the goal with for this system has been reducing the duration of mechanical ventilation, ICU time, as well as the risk for chest wall deformities.
Although the majority of cases with fractured ribs can adequately be treated non-surgically, the remaining number particularly severe chest wall trauma cases can be a cause of morbidity and mortality, especially in the presence of a flail chest where paradoxical inward movement of the flail segment in inspiration is found. About 10% of chest wall trauma cases result in a flail chest. Flail chest injuries, defined as fracture of at least three consecutive ribs in at least two locations each, are associated with a mortality rate of up to 36%.1
Implants and screws
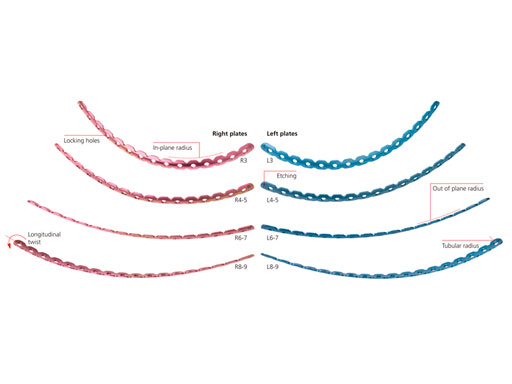
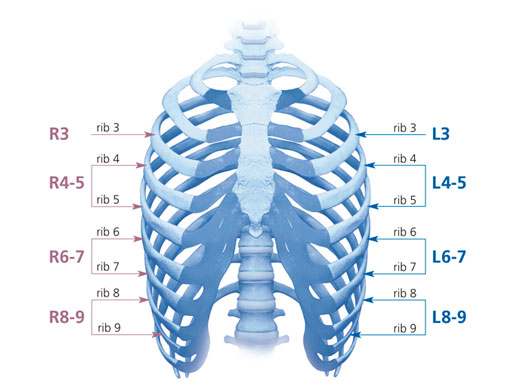
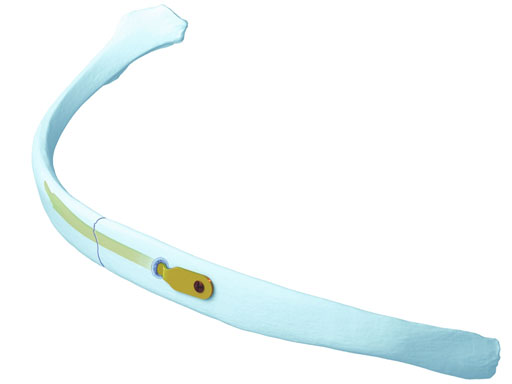
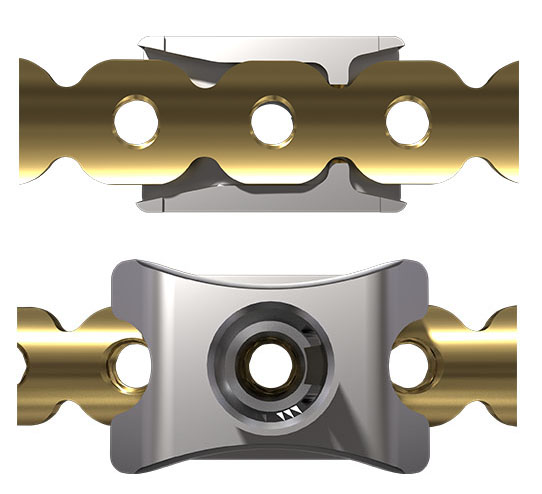
The MatrixRIB pre-contoured plates are available in sets of four (four left plates and four right plates, see Fig 2a) with designs that correspond to a specific rib or rib pair (Fig 2b). The plates, which cover fractures in all ribs suitable for plating, were designed to accommodate anatomic similarities between specific ribs.
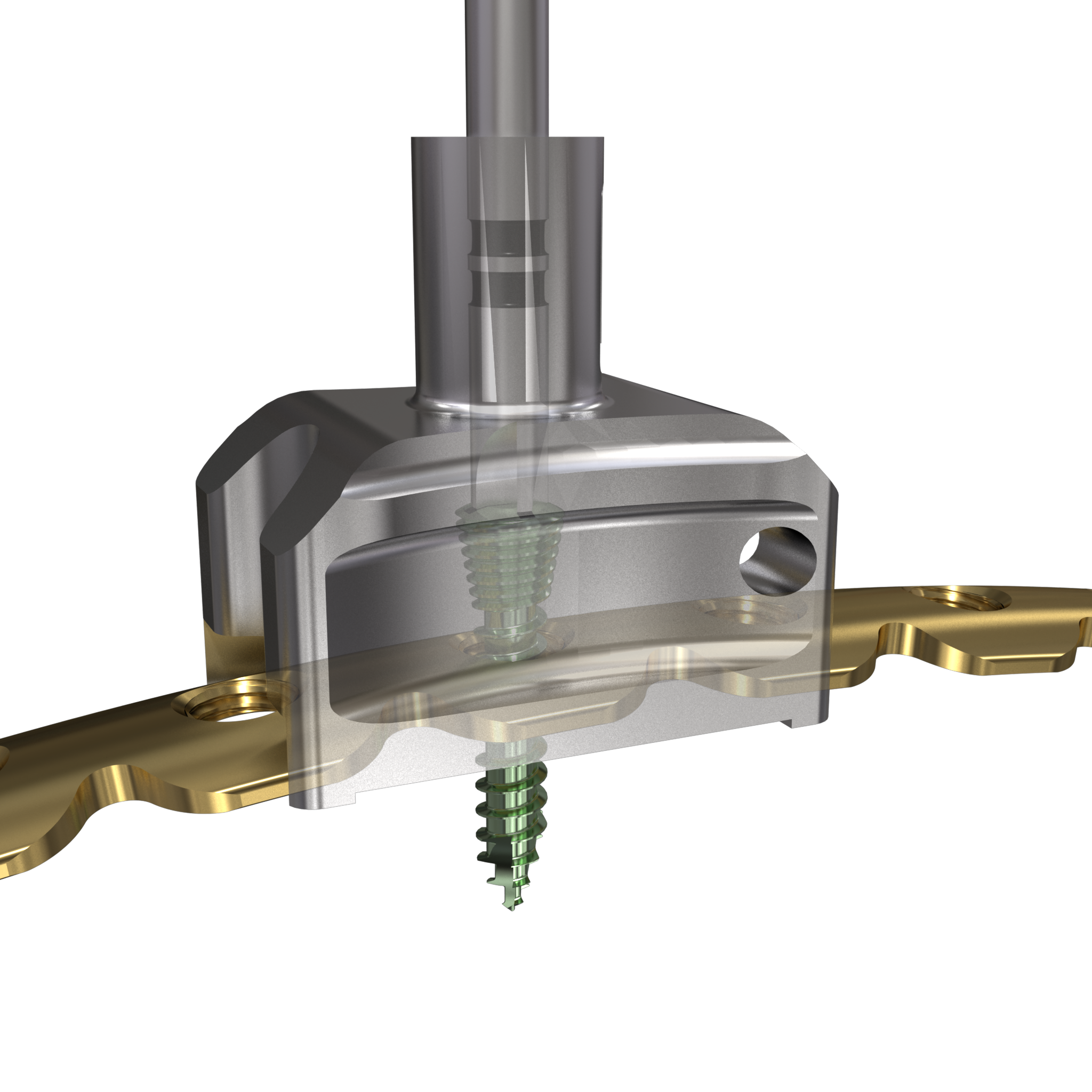
The MatrixRIB Fixation System is used with 2.9 mm self-tapping, locking cortex screws ranging from 6 to 14 mm in length (in increments of 2 mm). These screws are designed to be used with both the Synthes rib plates and intramedullary rib splints. The screws are made of titanium alloy (Ti-6Al- 7Nb). A 6 mm non-locking screw is also available to temporarily secure the plate during insertion of the locking screws. This non-locking screw is intended to be removed and replaced with a locking screw prior to soft-tissue closure.
The fixation of plates and screws can be done in the standard fashion, drilling of holes should be done most cautiously to avoid the risk of pneumothorax.
Splints
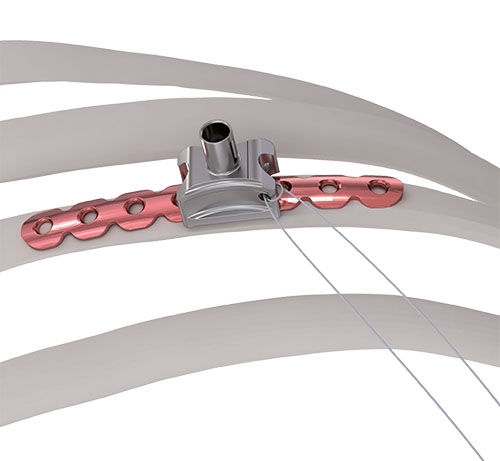
In addition to the plates, the MatrixRIB Fixation System includes intramedullary rib splints for fixation and stabilization of rib fractures especially on the posterior side in a minimally-invasive fashion. The rib splints have a rectangular cross-section, provide bicortical screw fixation, and are available in three widths: Small (3 mm), Medium (4 mm), and Large (5 mm).
For their implementation, specifically designed drill guides with hook as well as templates to prepare the canal are available.
Chest Wall Deformity Reconstruction System
In May 2015, the MatrixRIB long straight plates 24- and 30-hole were launched as part of the Chest Wall Deformity Reconstruction System (Fig 4a). Shortly after we have witnessed the launch of the new sternal plates (Fig 4b & 4c) which complete the system and provide users with a comprehensive and varied portfolio for chest reconstruction.
New components added to the MatrixRIB Fixation System
The new self-drilling locking screws are 2.7 mm in diameter and available in 1 mm length increments from 8 mm to 20 mm. Self-drilling nonlocking screws are 2.7 mm in diameter and available in 10 mm and 12 mm lengths (Fig 5a). These are provided as alternatives to the existing MatrixRIB Self-Tapping locking and nonlocking screws. The pointed, cutting tip of the MatrixRIB Self-Drilling Screws enables surgeons to insert the screws without drilling a pilot hole. The new MatrixRIB Self-Drilling Screws are designed to lock the MatrixRIB plates 1.5 mm to create the same secure construct as the MatrixRIB Self-Tapping Screws. New screw guides are provided to ensure coaxial alignment of the self-drilling screws to the MatrixRIB plates 1.5 mm and MatrixRIB Splints, reducing the variability in screw alignment and orientation during insertion and ensuring construct locking strength (Fig 5b and Fig 5c). The Plate Screw Guide engages with the contours of the existing plate profile (Fig 5d). This Plate Screw Guide has also cut-outs on both ends that enable the surgeon to visually align to the adjacent screw holes. The MatrixRIB Self-Drilling Screws can be used in both the open and Minimally Invasive Plate Osteosynthesis (MIPO) approaches. If a suture is needed during the MIPO procedure, the guide has a through hole where a suture could be used as a tether (Fig 5e). The MatrixRIB Self-Drilling Screws are not meant to be used with the thick MatrixRIB plates 2.8 mm or in the 90° approach.
[1] Ciraulo DL, Elliott D, Mitchell KA, Rodriguez A. Flail chest as a marker for significant injuries. J Am Coll Surg. 1994 May;178(5):466-70.
Mohr M, Abrams E, Engel C, et al. Geometry of human ribs pertinent to orthopedic chestwall reconstruction. J Biomech 2007; 40: 1310-1317.
Instrumentation
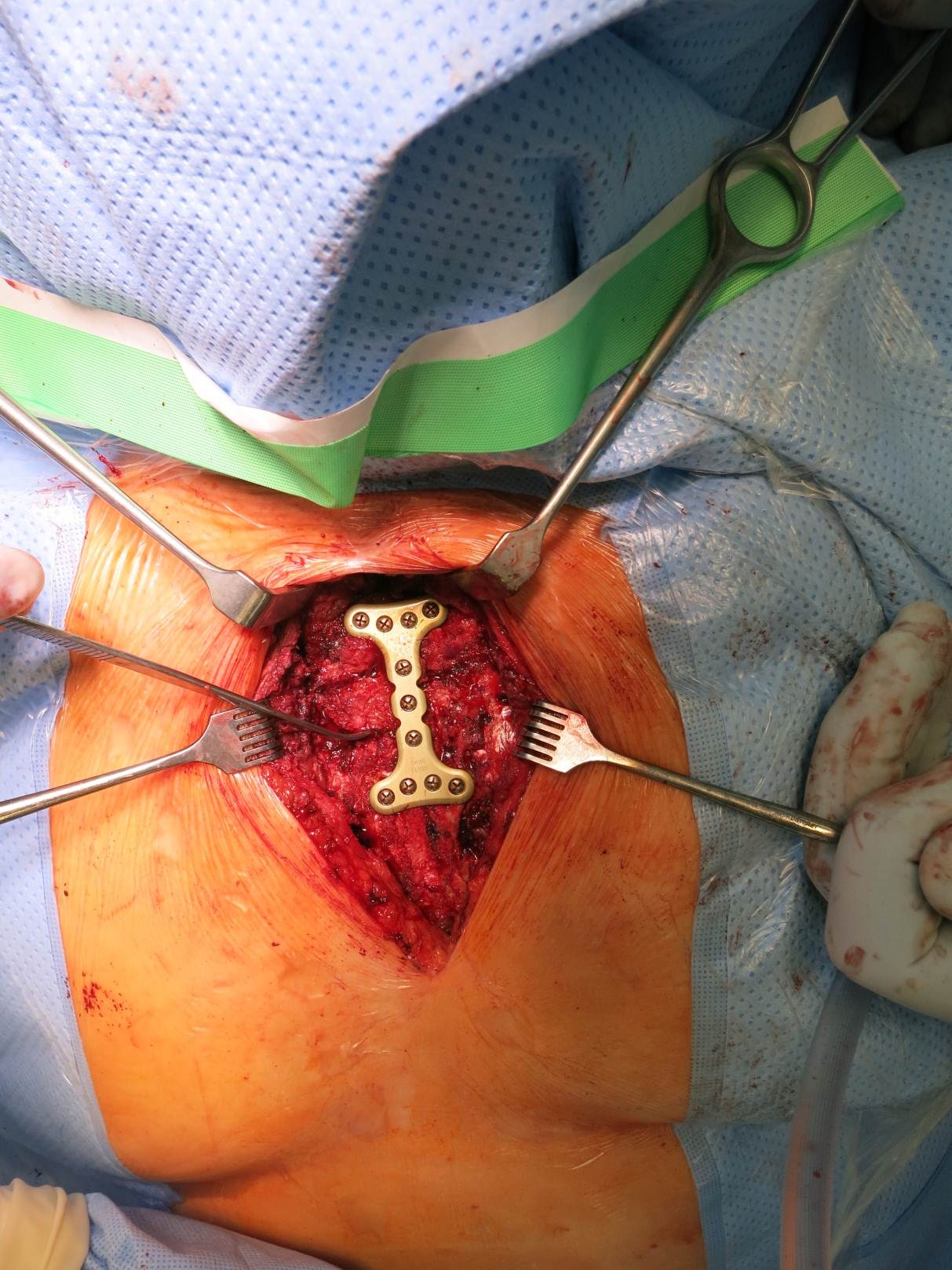
The specially developed instrumentation includes three plate holding forceps to assist the positioning of the plates during drilling and screw insertion procedures. These forceps are designed to be inserted from the superior aspect of the rib and hold the plate to the rib. The large version spans across multiple ribs to minimize the number of incisions in the intercostal space.
Case image provided by W Long, Portland, OR
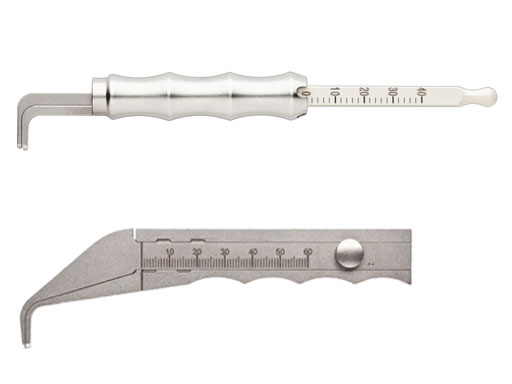
Caliper
The new universal caliper is designed for easy readability, durability, and compact size. In comparison with the currently available flat caliper this device also offers improved handling capabilities yet is easier to clean. The calibrated markings are etched in black on an off-white arm extension, which has a square shape and is etched on all four sides. The extensions arm is made of radel (polyphenylsulfone (PPSU)) for better durability. It is intended for various areas of thoracic surgery, ie, with the sternal fixation system and the matrix rib system.
Fall from 4-meter height
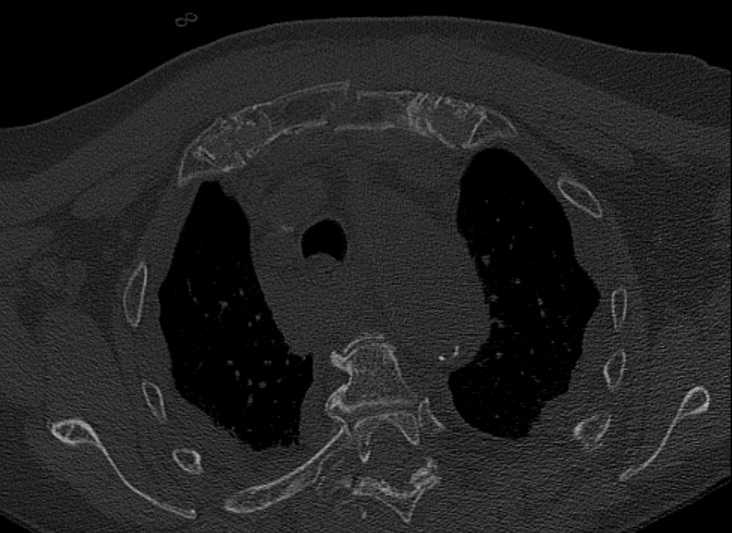
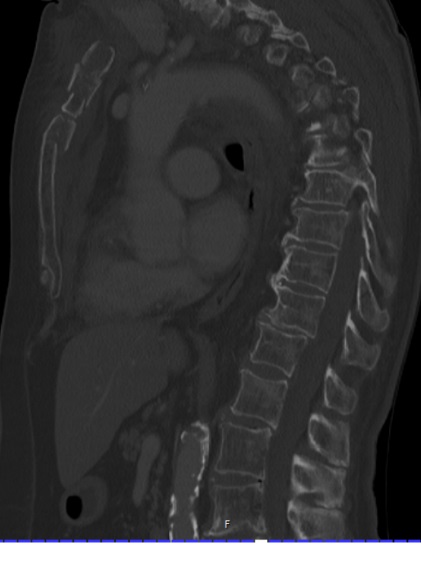
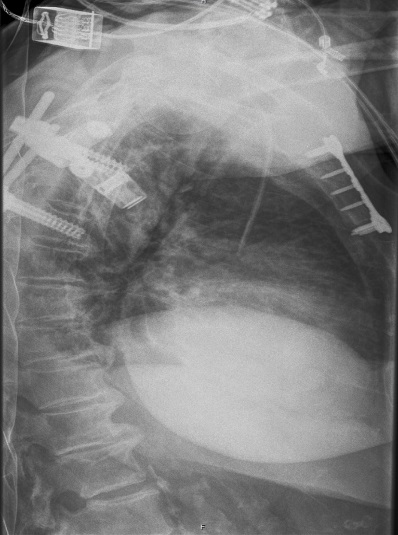
Mechanism of injury: An 81 year old woman fell onto her back from a height of 4 meters. (Fig 6a-d) She sustained a multiple sternal fracture concomitant with a fracture of the 5th thoracic vertebra resulting in an unstable injury of the trunk. Fortunately she did not show any neurologic deficit or paraplegia.
Diagnosis: Complex sternal fracture with multidirectional instability (longitudinal fracture of the manubrium, transverse fracture involving 3 levels of manubrium and corpus sterni) Concomitant to this was a fracture of the 5th thoracic vertebra (AOB2.1).
Treatment: The sternal fracture has been managed by open-reduction and internal fixation with a locked plate osteosynthesis (MatrixRIB, I-Plate) through an anterior approach in the mid-line. b The thoracic spine has been managed employing dorsal instrumentation (internal fixator th4-th6).
Post-operative outcome: The wounds and bone healed uneventfully. Breathing was possible spontaneously without any restrictions. The patient reported on a significant reduction of the pain immediately after the operative procedure. Mobilization on the ward started on the first day after the procedure. No complications such as secondary failure, non-unions, pain or deformity of the chest wall could be seen during the follow up examinations of 6 and 12 weeks and 6 and 12 months.
Pectus Excavatum
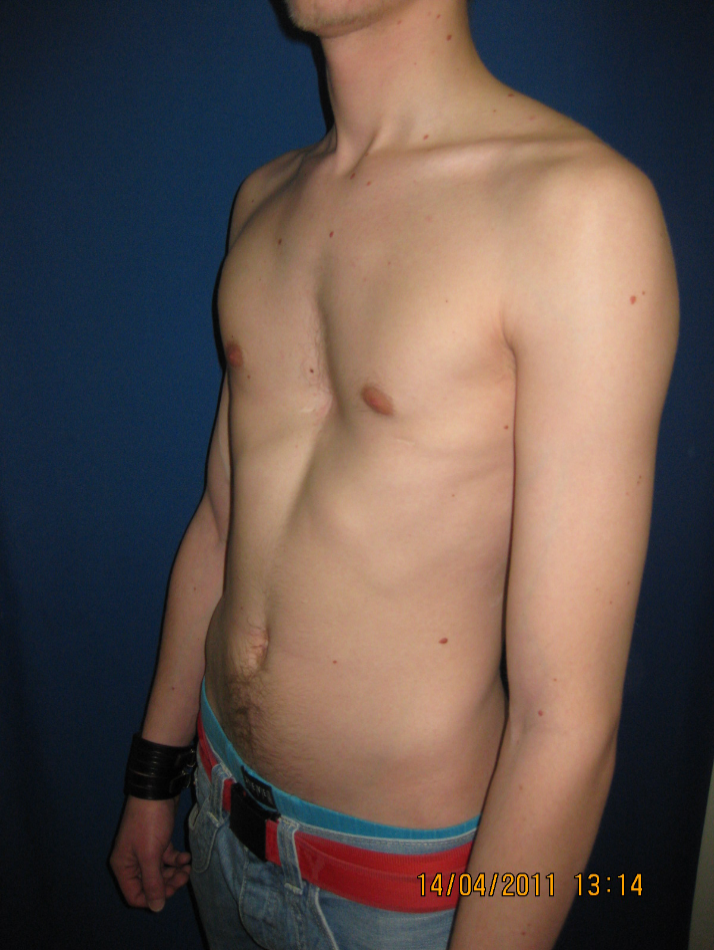
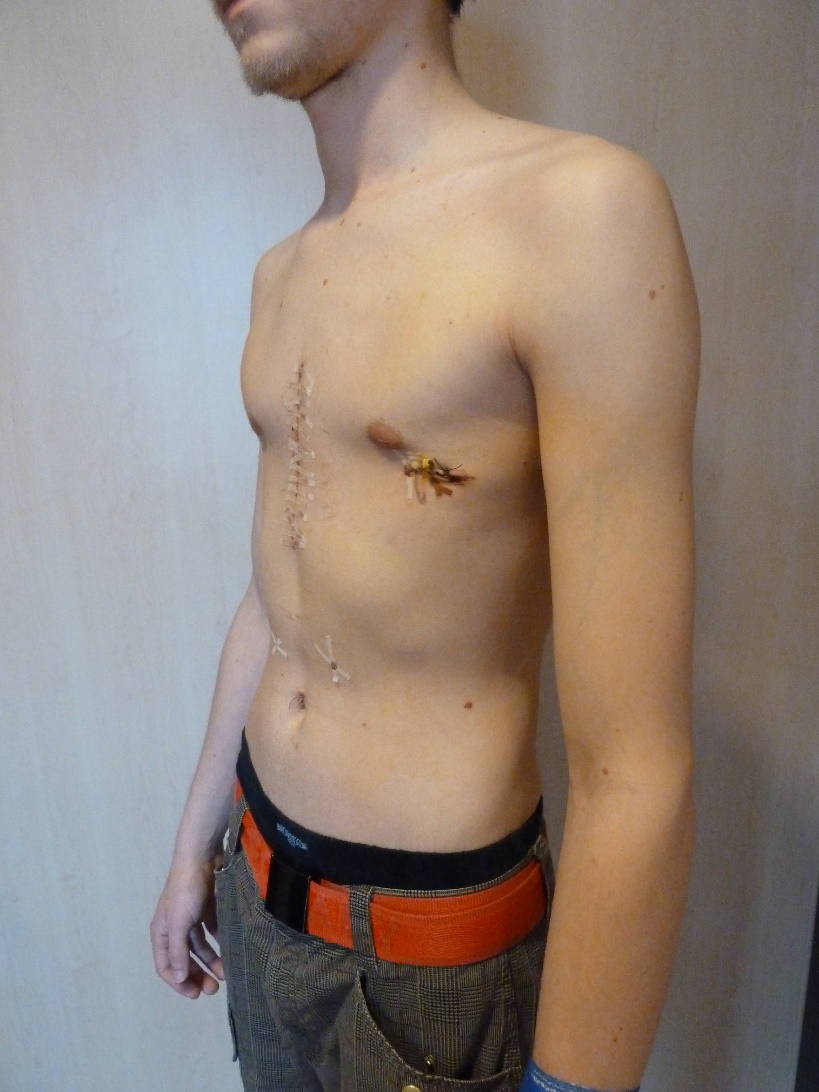
Clinical problem: A 21 year old male suffered from a recurrent pectus excavatum. Two years prior he underwent an open procedure to manage the deformity, including the insertion of one trans-sternal metal bar to stabilize the anterior chest wall (Fig 7a). Upon removal one year post-op, the anterior chest wall collapsed again and showed instability at the Sternocostal-junction involving ribs at the 4th 7th level.
Diagnosis: Recurrent pectus excavatum Unstable and dislocated Sternocostal-junction, 4-7th level.
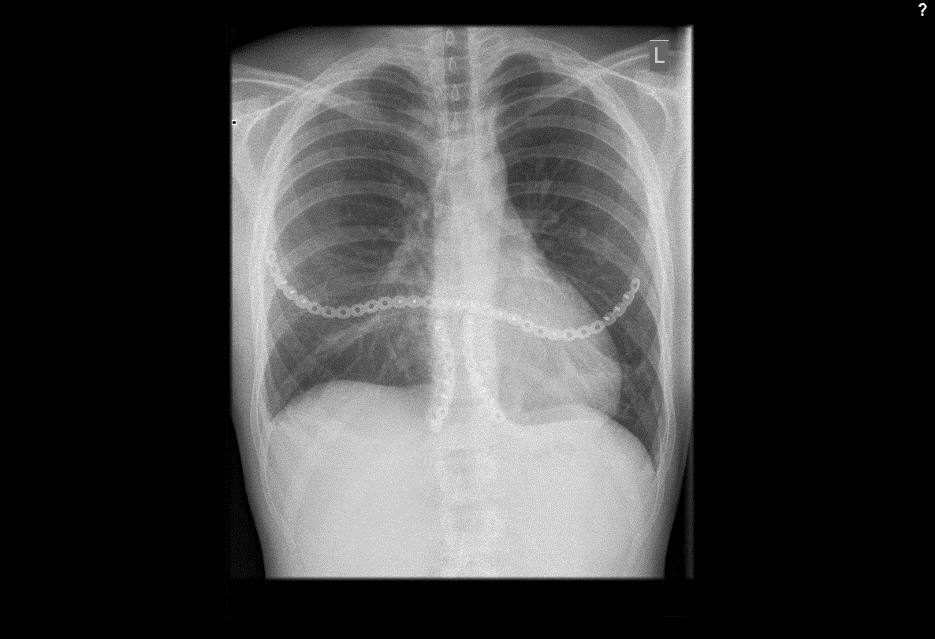
Treatment: Another open approach was necessary. The anterior chest wall was mobilized and stabilized with the MatrixRIB 30-hole straight plate adopting a rib to sternum to rib approach at the 4th level. A bilateral sternocostal fixation at the 7th level was also performed using the MatrixRIB 8-hole straight plate (Fig 7b). The transverse plate was fixed to the anterolateral aspect of the ribs through additional limited incisions bilaterally (Fig 7c). The 5th and 6th pair of ribs were reduced to the sternal level alongside the 7th rib. Strong sutures (PDS 1mm, BPT-3) were placed sternocostal to fix the ribs to the sternum.
The patient recovered well. Wound healing was uneventful. Both cosmetic and functional results were excellent. Mobilization could occur immediately after the operation. No complications have been seen in more than 5 years of follow up.
The treatment of chest wall deformity and pectus deformity using the MatrixRIB System
Minimal Invasive Rib Stabilization
Hazards and labeling
Due to varying countries’ legal and regulatory approval requirements, consult the appropriate local product labeling for approved intended use of the products described on this website. All devices on this website are approved by the AO Technical Commission. For logistical reasons, these devices may not be available in all countries worldwide at the date of publication.
Legal restrictions
This work was produced by AO Foundation, Switzerland. All rights reserved by AO Foundation. This publication, including all parts thereof, is legally protected by copyright.
Any use, exploitation or commercialization outside the narrow limits set forth by copyright legislation and the restrictions on use laid out below, without the publisher‘s consent, is illegal and liable to prosecution. This applies in particular to photostat reproduction, copying, scanning or duplication of any kind, translation, preparation of microfilms, electronic data processing, and storage such as making this publication available on Intranet or Internet.
Some of the products, names, instruments, treatments, logos, designs, etc referred to in this publication are also protected by patents, trademarks or by other intellectual property protection laws (eg, “AO” and the AO logo are subject to trademark applications/registrations) even though specific reference to this fact is not always made in the text. Therefore, the appearance of a name, instrument, etc without designation as proprietary is not to be construed as a representation by the publisher that it is in the public domain.
Restrictions on use: The rightful owner of an authorized copy of this work may use it for educational and research purposes only. Single images or illustrations may be copied for research or educational purposes only. The images or illustrations may not be altered in any way and need to carry the following statement of origin “Copyright by AO Foundation, Switzerland”.
Check www.aofoundation.org/disclaimer for more information.
If you have any comments or questions on the articles or the new devices, please do not hesitate to contact us.
“approved by AO Technical Commission” and “approved by AO”
The brands and labels “approved by AO Technical Commission” and “approved by AO”, particularly "AO" and the AO logo, are AO Foundation's intellectual property and subject to trademark applications and registrations, respectively. The use of these brands and labels is regulated by licensing agreements between AO Foundation and the producers of innovation products obliged to use such labels to declare the products as AO Technical Commission or AO Foundation approved solutions. Any unauthorized or inadequate use of these trademarks may be subject to legal action.
AO ITC Innovations Magazine
Find all issues of the AO ITC Innovations Magazine for download here.
Innovation Awards
Recognizing outstanding achievements in development and fostering excellence in surgical innovation.