6.5 mm Midfoot Fusion Bolt
Per-Henrik Agren, Andrew Sands
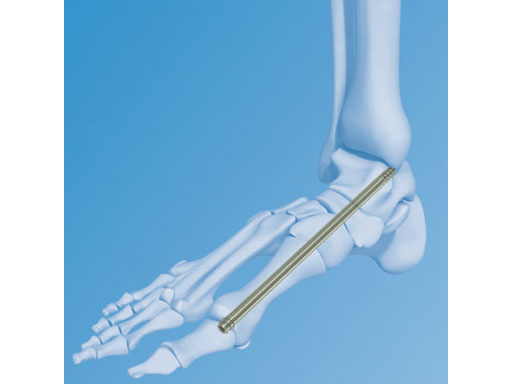
The Midfoot Fusion Bolt (MFB) is a solid 6.5 mm intramedullary implant that can be used to stabilize and fuse the medial columnthe metatarsocuneiform, naviculocuneiform, and talonavicular joints. Alternative uses of the MFB may also be for fusing the lateral column, calcaneocuboid, and 4th metatarsocuboid joint. The MFB aims to achieve permanent fusion of these joints in patients suffering from gross instability such as Charcot neuroarthropathy with or without collapse of the midfoot. The midfoot fusion bolt is a headless solid bolt. It enhances stabilization and alignment, restraining shearing and bending forces better than older implants (I-bolt, 6.5 mm cancelleous screw, and 6.5 mm and 7.3 mm cannulated screws). The MFB is recommended for stabilization of the medial column or fixation of the lateral column. The bones and joints are aligned, measured and drilled with cannulated instruments. The guide wire is removed. The bolt is inserted through the track created by the 5.0 mm drill. Controlled compression may be applied by the T-handle during insertion. After insertion, the bolt head is completely countersunk preventing soft tissue or joint irritation. A blunt tip prevents any damage to soft tissues if the MFB is overinserted.
There are situations where the normal architecture and biomechanics of the foot (specifically the medial column) becomes significantly altered. Gait disturbances, skin abnormalities, and ulcerations can develop leading to infections and even possible amputation. This may be painful if sensation is intact. Often, however, decreased sensation is part of the cause.
The causes include posttraumatic cases, inflammatory diseases such as rheumatoid arthritis, and rare problems such as Marfans disease or neuromuscular tertiary syphilis and paralytic diseases. A common reason today is the increasing population of patients with diabetic neuropathy sometimes causing neuroarthropathy.
These patients may be treated nonoperatively in an orthosis or total contact cast in an attempt to consolidate the unstable foot. However, this is a labor intensive and potentially costly process. The outcome is not always satisfactory.
If the initial treatment has been nonoperative, the further treatment often remains nonoperative. Some are ambulating in calipers and orthoses to relieve pressure from threatening ulcers, others in expensive customized shoes that they object to for cosmetic reasons. The quality of life is diminished immensely for someone with a Charcot foot, and also puts a high cost on health care systems.
A possible solution for pressure points is the bumpectomy (local resection of protruding bone with no stabilization). If the joint segments are still unstable (the more common situation) the deformity will however progress.
This is why a reconstructive realignment usually is necessary to achieve a more normal weight bearing and alignment of the foot.
The first recognized success of such treatment was in the early 1990s in Seattle by Sigvard T Hansen, Jr. In many cases, the patient had a longstanding pressure wound on the plantar surface of the foot. Reconstruction was carried out in the presence of an open ulcer, which was covered by a biodressing during the procedure on the rest of the foot. After surgical reconstruction removed the unnatural pressure on the soft tissues, the ulcer disappeared within a few weeks.
Even very heavy/thick screws fail in these patients (especially as the patients are getting heavier with time). The 6.5mm Midfoot Fudion Bolt has been developed specifically to treat the forces and demands in the midfoot area.
The MFB is available in lengths of 50160 mm in titanium and stainless steel.
The Foot and Ankle Expert Group wanted the MFB to be a simple, strong, easy-to-use implant because Charcot foot collpase will be more prevalent over the coming years. First, it needs to be said that it is not meant as a compressive screw. The compression should be achieved by other implantsscrews or plates that are added. The threads on the MFB are intended to keep the bolt where it is positioned.
The standard approach to release the midfoot and to produce the necessary corrections is the medial utility incision. It can be extended all along the medial foot from medial malleolus region to the medial hallux. There are several options for the introduction of the implant along the medial column:
- The main track is through the MTP1 transarticularly. With minimal dissection the cannulated drilling and MFB insertion is done with the great toe flexed downwards.
- A variant is to perform a distal osteotomy shift the head of MT1 laterally (chevron-type) and perform the implantation (adapted in a patient with sensation).
- A third variant is the retroantegrade techniquedrilling over the wire in an antegrade fashionintroducing the screw the same way. This lessens the damage in MTP 1 but harms the ankle instead.
On the lateral side of the foot the drill wire is passed through MT 4 cuboid and out through calcaneus while the foot is maintained in the desired alignment. The wire is then overdrilled antegrade from back to front and the bolt is inserted.
The entry point in MT 4 is 22,5 cm from the TMT joint to achieve some grip with the bolt.
The postoperative treatment is immobilization in a splint for 2 weeks to reduce swelling. At 2 weeks, the wound is evaluated and gentle range of motion training of joints is initiated. Full weight bearing is anticipated between 1012 weeks postop based on radiographical evidence of healing.
Producing a stable construct before the foot has progressed to an advanced deformity, when the start of the breakdown is detected, may lead to a better, more reliable result with a better quality of life for these patients. It is also anticipated to lower the high costs of healthcare in diabetic foot clinics and for amputations and prostheses.
There is data in literature in this direction and therefore a randomizedcontrolled multicenter study run by AO Clinical Investigation and Documentation (CID) is being initiated to compare the effectiveness of surgical arthrodesis with MFB in the early stage of diabetic-neuropathic Charcot feet. This will be compared to nonoperative treatment in terms of maintenance of the correction of the deformity and avoidance of permanent nonreducible foot deformity.
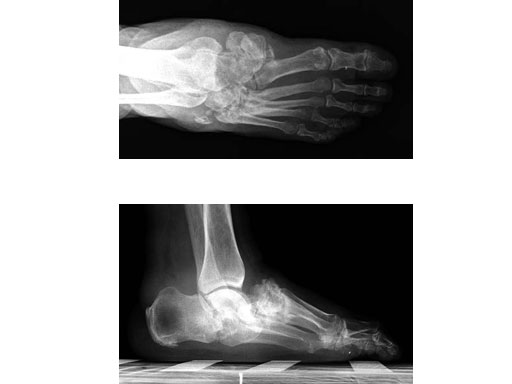
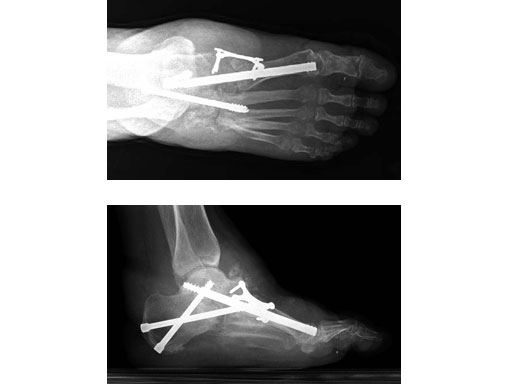
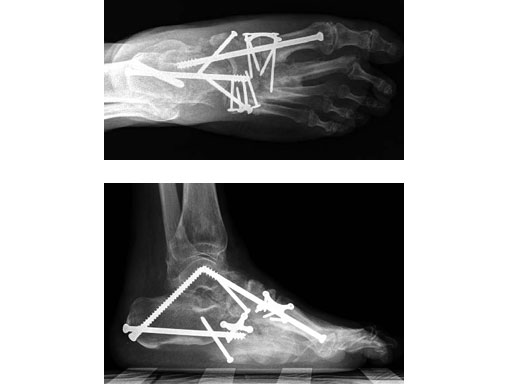
Case 1: 47-year-old female
Case provided by Andrew K Sands, New York, USA
Charcot foot treated with MFB and X-plate
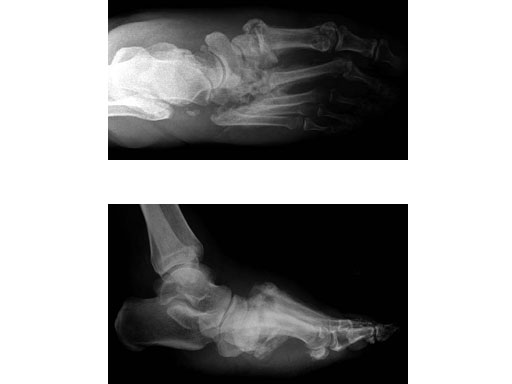
Case 2: 48-year-old male
Case provided by Andrew K Sands, New York, USA
Charcot foot treated with MFB and X-plate.
Hazards and labeling
Due to varying countries’ legal and regulatory approval requirements, consult the appropriate local product labeling for approved intended use of the products described on this website. All devices on this website are approved by the AO Technical Commission. For logistical reasons, these devices may not be available in all countries worldwide at the date of publication.
Legal restrictions
This work was produced by AO Foundation, Switzerland. All rights reserved by AO Foundation. This publication, including all parts thereof, is legally protected by copyright.
Any use, exploitation or commercialization outside the narrow limits set forth by copyright legislation and the restrictions on use laid out below, without the publisher‘s consent, is illegal and liable to prosecution. This applies in particular to photostat reproduction, copying, scanning or duplication of any kind, translation, preparation of microfilms, electronic data processing, and storage such as making this publication available on Intranet or Internet.
Some of the products, names, instruments, treatments, logos, designs, etc referred to in this publication are also protected by patents, trademarks or by other intellectual property protection laws (eg, “AO” and the AO logo are subject to trademark applications/registrations) even though specific reference to this fact is not always made in the text. Therefore, the appearance of a name, instrument, etc without designation as proprietary is not to be construed as a representation by the publisher that it is in the public domain.
Restrictions on use: The rightful owner of an authorized copy of this work may use it for educational and research purposes only. Single images or illustrations may be copied for research or educational purposes only. The images or illustrations may not be altered in any way and need to carry the following statement of origin “Copyright by AO Foundation, Switzerland”.
Check www.aofoundation.org/disclaimer for more information.
If you have any comments or questions on the articles or the new devices, please do not hesitate to contact us.
“approved by AO Technical Commission” and “approved by AO”
The brands and labels “approved by AO Technical Commission” and “approved by AO”, particularly "AO" and the AO logo, are AO Foundation's intellectual property and subject to trademark applications and registrations, respectively. The use of these brands and labels is regulated by licensing agreements between AO Foundation and the producers of innovation products obliged to use such labels to declare the products as AO Technical Commission or AO Foundation approved solutions. Any unauthorized or inadequate use of these trademarks may be subject to legal action.
AO ITC Innovations Magazine
Find all issues of the AO ITC Innovations Magazine for download here.
Innovation Awards
Recognizing outstanding achievements in development and fostering excellence in surgical innovation.